Faster decay of neutralizing antibodies in never infected than previously infected healthcare workers three months after the second BNT162b2 mRNA COVID-19 vaccine dose
- PMID: 34481967
- PMCID: PMC8410637
- DOI: 10.1016/j.ijid.2021.08.052
Faster decay of neutralizing antibodies in never infected than previously infected healthcare workers three months after the second BNT162b2 mRNA COVID-19 vaccine dose
Abstract
Objectives: This study aimed to describe the longitudinal evolution of neutralizing antibody titres (NtAb) in three different cohorts of healthcare workers (HCWs), including vaccinated HCWs with and without a previous SARS-CoV-2 infection and previously infected unvaccinated HCWs. COVID-19 was mild or asymptomatic in those experiencing infection.
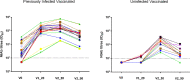
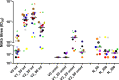
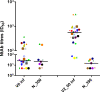
Methods: NtAb was tested before BNT162b2 mRNA COVID-19 vaccine (V0), 20±2 days after the first dose (V1_20), 20±3 days (V2_20) and 90±2 days (V2_90) after the second dose in vaccinated HCWs and after about 2 months (N_60), 10 months (N_300) and 13 months (N_390) from natural infection in unvaccinated HCWs. NtAb were measured by authentic virus neutralization with a SARS-CoV-2 B.1 isolate circulating in Italy at HCW enrolment.
Results: Sixty-two HCWs were enrolled. NtAb were comparable in infected HCWs with no or mild disease at all the study points. NtAb of uninfected HCWs were significantly lower with respect to those of previously infected HCWs at V1_20, V2_20 and V2_90. The median NtAb fold decrease from V2_20 to V2_90 was higher in the uninfected HCWs with respect to those with mild infection (6.26 vs 2.58, p=0.03) and to asymptomatic HCWs (6.26 vs 3.67, p=0.022). The median Nabt at N_390 was significantly lower than at N_60 (p=0.007).
Conclusions: In uninfected HCWs completing the two-dose vaccine schedule, a third mRNA vaccine dose is a reasonable option to counteract the substantial NtAb decline occurring at a significantly higher rate compared with previously infected, vaccinated HCWs. Although low, Nabt were still at a detectable level after 13 months in two-thirds of previously infected and unvaccinated HCWs.
Keywords: Asymptomatic; BNT162b2 mRNA COVID-19 vaccine; COVID-19; Healthcare workers; Mild disease; Neutralizing antibodies.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interests The authors declare that they have no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

