Simulating the time evolving geometry, mechanical properties, and fibrous structure of bioprosthetic heart valve leaflets under cyclic loading
- PMID: 34482092
- PMCID: PMC8482999
- DOI: 10.1016/j.jmbbm.2021.104745
Simulating the time evolving geometry, mechanical properties, and fibrous structure of bioprosthetic heart valve leaflets under cyclic loading
Abstract
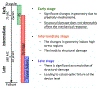
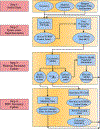
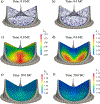
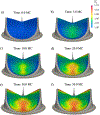
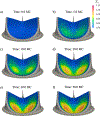
Currently, the most common replacement heart valve design is the 'bioprosthetic' heart valve (BHV), which has important advantages in that it does not require permanent anti-coagulation therapy, operates noiselessly, and has blood flow characteristics similar to the native valve. BHVs are typically fabricated from glutaraldehyde-crosslinked pericardial xenograft tissue biomaterials (XTBs) attached to a rigid, semi-flexible, or fully collapsible stent in the case of the increasingly popular transcutaneous aortic valve replacement (TAVR). While current TAVR assessments are positive, clinical results to date are generally limited to <2 years. Since TAVR leaflets are constructed using thinner XTBs, their mechanical demands are substantially greater than surgical BHV due to the increased stresses during in vivo operation, potentially resulting in decreased durability. Given the functional complexity of heart valve operation, in-silico predictive simulations clearly have potential to greatly improve the TAVR development process. As such simulations must start with accurate material models, we have developed a novel time-evolving constitutive model for pericardial xenograft tissue biomaterials (XTB) utilized in BHV (doi: 10.1016/j.jmbbm.2017.07.013). This model was able to simulate the observed tissue plasticity effects that occur in approximately in the first two years of in vivo function (50 million cycles). In the present work, we implemented this model into a complete simulation pipeline to predict the BHV time evolving geometry to 50 million cycles. The pipeline was implemented within an isogeometric finite element formulation that directly integrated our established BHV NURBS-based geometry (doi: 10.1007/s00466-015-1166-x). Simulations of successive loading cycles indicated continual changes in leaflet shape, as indicated by spatially varying increases in leaflet curvature. While the simulation model assumed an initial uniform fiber orientation distribution, anisotropic regional changes in leaflet tissue plastic strain induced a complex changes in regional fiber orientation. We have previously noted in our time-evolving constitutive model that the increases in collagen fiber recruitment with cyclic loading placed an upper bound on plastic strain levels. This effect was manifested by restricting further changes in leaflet geometry past 50 million cycles. Such phenomena was accurately captured in the valve-level simulations due to the use of a tissue-level structural-based modeling approach. Changes in basic leaflet dimensions agreed well with extant experimental studies. As a whole, the results of the present study indicate the complexity of BHV responses to cyclic loading, including changes in leaflet shape and internal fibrous structure. It should be noted that the later effect also influences changes in local mechanical behavior (i.e. changes in leaflet anisotropic tissue stress-strain relationship) due to internal fibrous structure resulting from plastic strains. Such mechanism-based simulations can help pave the way towards the application of sophisticated simulation technologies in the development of replacement heart valve technology.
Keywords: Bioprosthetic heart valve; Simulation; Soft tissue mechanics; Time evolving properties.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



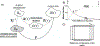









References
-
- Schoen FJ, Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering, Circulation 118 (18) (2008) 1864–80. doi: 10.1161/CIRCULATIONAHA.108.805911. URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop... - DOI - PubMed
-
- Schoen FJ, New frontiers in the pathology and therapy of heart valve disease: 2006 Society for Cardiovascular Pathology, Distinguished Achievement Award Lecture, United States-Canadian Academy of Pathology, Atlanta, GA, February 12, 2006, Cardiovasc Pathol 15 (5) (2006) 271–9. URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop... - PubMed
-
- Vesely I, Barber JE, Ratliff NB, Tissue damage and calcification may be independent mechanisms of bioprosthetic heart valve failure, J Heart Valve Dis 10 (4) (2001) 471–7. URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop... - PubMed
-
- Sacks MS, Schoen FJ, Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves, J Biomed Mater Res 62 (3) (2002) 359–71. URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop... - PubMed
-
- Schoen F, Levy R, Tissue heart valves: Current challenges and future research perspectives, Journal of Biomedical Materials Research 47 (1999) 439–465. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

