Translational Strategies for Repotrectinib in Neuroblastoma
- PMID: 34482287
- PMCID: PMC8571000
- DOI: 10.1158/1535-7163.MCT-21-0126
Translational Strategies for Repotrectinib in Neuroblastoma
Abstract
Limited clinical data are available regarding the utility of multikinase inhibition in neuroblastoma. Repotrectinib (TPX-0005) is a multikinase inhibitor that targets ALK, TRK, JAK2/STAT, and Src/FAK, which have all been implicated in the pathogenesis of neuroblastoma. We evaluated the preclinical activity of repotrectinib monotherapy and in combination with chemotherapy as a potential therapeutic approach for relapsed/refractory neuroblastoma. In vitro sensitivity to repotrectinib, ensartinib, and cytotoxic chemotherapy was evaluated in neuroblastoma cell lines. In vivo antitumor effect of repotrectinib monotherapy, and in combination with chemotherapy, was evaluated using a genotypically diverse cohort of patient-derived xenograft (PDX) models of neuroblastoma. Repotrectinib had comparable cytotoxic activity across cell lines irrespective of ALK mutational status. Combination with chemotherapy demonstrated increased antiproliferative activity across several cell lines. Repotrectinib monotherapy had notable antitumor activity and prolonged event-free survival compared with vehicle and ensartinib in PDX models (P < 0.05). Repotrectinib plus chemotherapy was superior to chemotherapy alone in ALK-mutant and ALK wild-type PDX models. These results demonstrate that repotrectinib has antitumor activity in genotypically diverse neuroblastoma models, and that combination of a multikinase inhibitor with chemotherapy may be a promising treatment paradigm for translation to the clinic.
©2021 American Association for Cancer Research.
Figures


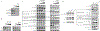
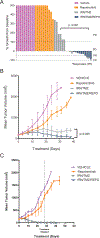
References
-
- Howlader NA, Krapcho M, et al.. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) Bethesda, MD. National Cancer Institute; 2012.
-
- De Brouwer S, De Preter K, Kumps C, Zabrocki P, Porcu M, Westerhout EM, et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clinical cancer research : an official journal of the American Association for Cancer Research 2010;16(17):4353–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

