Intraluminal diamond-like carbon coating with anti-adhesion and anti-biofilm effects for uropathogens: A novel technology applicable to urinary catheters
- PMID: 34482564
- PMCID: PMC9290946
- DOI: 10.1111/iju.14675
Intraluminal diamond-like carbon coating with anti-adhesion and anti-biofilm effects for uropathogens: A novel technology applicable to urinary catheters
Abstract
Objectives: To examine anti-adhesion and anti-biofilm effects of a diamond-like carbon coating deposited via a novel technique on the inner surface of a thin silicon tube.
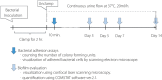
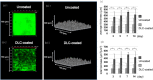
Methods: Diamond-like carbon coatings were deposited into the lumen of a silicon tube with inner diameters of 2 mm. The surface of the diamond-like carbon was evaluated using physicochemical methods. We used three clinical isolates including green fluorescent protein-expressing Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus. We employed a continuous flow system for evaluation of both bacterial adhesion and biofilm formation. Bacterial adhesion assays consisted of counting the number of colony-forming units and visualization of adhered bacterial cells by scanning electron microscope to evaluate the diamond-like carbon-coated/uncoated samples. The biofilm structure was analyzed by confocal laser scanning microscopy on days 3, 5, 7 and 14 for green fluorescent protein-expressing Pseudomonas aeruginosa.
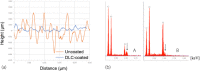
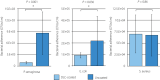
Results: The smooth and carbon-rich structure of the intraluminal diamond-like carbon film remained unchanged after the experiments. The numbers of colony-forming units suggested lower adherence of green fluorescent protein-expressing Pseudomonas aeruginosa and Escherichia coli in the diamond-like carbon-coated samples compared with the uncoated samples. The scanning electron microscope images showed adhered green fluorescent protein-expressing Pseudomonas aeruginosa cells without formation of microcolonies on the diamond-like carbon-coated samples. Finally, biofilm formation on the diamond-like carbon-coated samples was lower until at least day 14 compared with the uncoated samples.
Conclusions: Intraluminal diamond-like carbon coating on a silicone tube has anti-adhesion and anti-biofilm effects. This technology can be applied to urinary catheters made from various materials.
Keywords: bacterial adhesion; biofilms; plasma gases; urinary catheters; urinary tract infection.
© 2021 The Authors. International Journal of Urology published by John Wiley & Sons Australia, Ltd on behalf of the Japanese Urological Association.
Conflict of interest statement
None declared.
Figures




Comment in
-
Editorial Comment to Intraluminal diamond-like carbon coating with anti-adhesion and anti-biofilm effects for uropathogens: A novel technology applicable to urinary catheters.Int J Urol. 2021 Dec;28(12):1289. doi: 10.1111/iju.14691. Epub 2021 Sep 7. Int J Urol. 2021. PMID: 34494311 No abstract available.
Similar articles
-
Diamond-like carbon coating to inner surface of polyurethane tube reduces Staphylococcus aureus bacterial adhesion and biofilm formation.J Artif Organs. 2024 Jun;27(2):108-116. doi: 10.1007/s10047-023-01403-1. Epub 2023 May 25. J Artif Organs. 2024. PMID: 37227545 Free PMC article.
-
Editorial Comment to Intraluminal diamond-like carbon coating with anti-adhesion and anti-biofilm effects for uropathogens: A novel technology applicable to urinary catheters.Int J Urol. 2021 Dec;28(12):1289. doi: 10.1111/iju.14691. Epub 2021 Sep 7. Int J Urol. 2021. PMID: 34494311 No abstract available.
-
Urinary Catheters Coated with a Novel Biofilm Preventative Agent Inhibit Biofilm Development by Diverse Bacterial Uropathogens.Antibiotics (Basel). 2022 Oct 30;11(11):1514. doi: 10.3390/antibiotics11111514. Antibiotics (Basel). 2022. PMID: 36358169 Free PMC article.
-
Diamond-like carbon coatings on ureteral stents--a new strategy for decreasing the formation of crystalline bacterial biofilms?J Urol. 2007 May;177(5):1923-7. doi: 10.1016/j.juro.2007.01.016. J Urol. 2007. PMID: 17437849
-
Effect of vancomycin-coated tympanostomy tubes on methicillin-resistant Staphylococcus aureus biofilm formation: in vitro study.J Laryngol Otol. 2010 Jun;124(6):594-8. doi: 10.1017/S0022215109992672. Epub 2010 Jan 8. J Laryngol Otol. 2010. PMID: 20056010 Review.
Cited by
-
Development of Antimicrobial Surfaces Using Diamond-like Carbon or Diamond-like Carbon-Based Coatings.Int J Mol Sci. 2024 Aug 6;25(16):8593. doi: 10.3390/ijms25168593. Int J Mol Sci. 2024. PMID: 39201280 Free PMC article. Review.
-
A novel antibacterial and fluorescent coating composed of polydopamine and carbon dots on the surface of orthodontic brackets.J Mater Sci Mater Med. 2023 Feb 21;34(2):10. doi: 10.1007/s10856-023-06712-8. J Mater Sci Mater Med. 2023. PMID: 36802301 Free PMC article.
-
Diamond-like carbon coating to inner surface of polyurethane tube reduces Staphylococcus aureus bacterial adhesion and biofilm formation.J Artif Organs. 2024 Jun;27(2):108-116. doi: 10.1007/s10047-023-01403-1. Epub 2023 May 25. J Artif Organs. 2024. PMID: 37227545 Free PMC article.
-
A review on antimicrobial strategies in mitigating biofilm-associated infections on medical implants.Curr Res Microb Sci. 2024 Mar 7;6:100231. doi: 10.1016/j.crmicr.2024.100231. eCollection 2024. Curr Res Microb Sci. 2024. PMID: 38510214 Free PMC article. Review.
-
Modification of SWCNTs with hybrid materials ZnO-Ag and ZnO-Au for enhancing bactericidal activity of phagocytic cells against Escherichia coli through NOX2 pathway.Sci Rep. 2022 Oct 13;12(1):17203. doi: 10.1038/s41598-022-22193-1. Sci Rep. 2022. PMID: 36229515 Free PMC article.
References
-
- Venkatesan N, Shroff S, Jayachandran K, Doble M. Polymers as ureteral stents. J. Endourol. 2010; 24: 191–8. - PubMed
-
- Paick SH, Park HK, Oh SJ, Kim HH. Characteristics of bacterial colonization and urinary tract infection after indwelling of double‐J ureteral stent. Urology 2003; 62: 214–7. - PubMed
-
- Bonfill X, Rigau D, Esteban‐Fuertes M et al. Efficacy and safety of urinary catheters with silver alloy coating in patients with spinal cord injury: a multicentric pragmatic randomized controlled trial. The ESCALE trial. Spine J. 2017; 17: 1650–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

