Reciprocal epigenetic remodeling controls testicular cancer hypersensitivity to hypomethylating agents and chemotherapy
- PMID: 34482638
- PMCID: PMC8807365
- DOI: 10.1002/1878-0261.13096
Reciprocal epigenetic remodeling controls testicular cancer hypersensitivity to hypomethylating agents and chemotherapy
Abstract
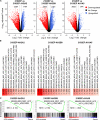
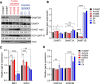
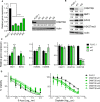
Testicular germ cell tumors (TGCTs) are aggressive but sensitive to cisplatin-based chemotherapy. Alternative therapies are needed for tumors refractory to cisplatin with hypomethylating agents providing one possibility. The mechanisms of cisplatin hypersensitivity and resistance in TGCTs remain poorly understood. Recently, it has been shown that TGCTs, even those resistant to cisplatin, are hypersensitive to very low doses of hypomethylating agents including 5-aza deoxy-cytosine (5-aza) and guadecitabine. We undertook a pharmacogenomic approach in order to better understand mechanisms of TGCT hypomethylating agent hypersensitivity by generating a panel of acquired 5-aza-resistant TGCT cells and contrasting these to previously generated acquired isogenic cisplatin-resistant cells from the same parent. Interestingly, there was a reciprocal relationship between cisplatin and 5-aza sensitivity, with cisplatin resistance associated with increased sensitivity to 5-aza and 5-aza resistance associated with increased sensitivity to cisplatin. Unbiased transcriptome analysis revealed 5-aza-resistant cells strongly downregulated polycomb target gene expression, the exact opposite of the finding for cisplatin-resistant cells, which upregulated polycomb target genes. This was associated with a dramatic increase in H3K27me3 and decrease in DNMT3B levels in 5-aza-resistant cells, the exact opposite changes seen in cisplatin-resistant cells. Evidence is presented that reciprocal regulation of polycomb and DNMT3B may be initiated by changes in DNMT3B levels as DNMT3B knockdown alone in parental cells resulted in increased expression of H3K27me3, EZH2, and BMI1, conferred 5-aza resistance and cisplatin sensitization, and mediated genome-wide repression of polycomb target gene expression. Finally, genome-wide analysis revealed that 5-aza-resistant, cisplatin-resistant, and DNMT3B-knockdown cells alter the expression of a common set of polycomb target genes. This study highlights that reciprocal epigenetic changes mediated by DNMT3B and polycomb may be a key driver of the unique cisplatin and 5-aza hypersensitivity of TGCTs and suggests that distinct epigenetic vulnerabilities may exist for pharmacological targeting of TGCTs.
Keywords: 5-aza deoxycytidine; DNA methylation; H3K27me3; cisplatin; epigenetics; polycomb repressive complex.
© 2021 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA & Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424. - PubMed
-
- Adra N & Einhorn LH (2017) Testicular cancer update. Clin Adv Hematol Oncol 15, 386–396. - PubMed
-
- Feldman DR, Patil S, Trinos MJ, Carousso M, Ginsberg MS, Sheinfeld J, Bajorin DF, Bosl GJ & Motzer RJ (2012) Progression‐free and overall survival in patients with relapsed/refractory germ cell tumors treated with single‐agent chemotherapy: endpoints for clinical trial design. Cancer 118, 981–986. - PubMed
-
- Biswal BK, Beyrouthy MJ, Hever‐Jardine MP, Armstrong D, Tomlinson CR, Christensen BC, Marsit CJ & Spinella MJ (2012) Acute hypersensitivity of pluripotent testicular cancer‐derived embryonal carcinoma to low‐dose 5‐aza deoxycytidine is associated with global DNA damage‐associated p53 activation, anti‐pluripotency and DNA demethylation. PLoS One 7, e53003. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

