Cleavage of E-cadherin by porcine respiratory bacterial pathogens facilitates airway epithelial barrier disruption and bacterial paracellular transmigration
- PMID: 34482810
- PMCID: PMC8425755
- DOI: 10.1080/21505594.2021.1966996
Cleavage of E-cadherin by porcine respiratory bacterial pathogens facilitates airway epithelial barrier disruption and bacterial paracellular transmigration
Abstract
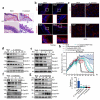
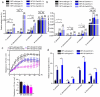
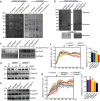
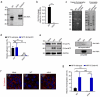
Airway epithelial cells are the first line of defense against respiratory pathogens. Porcine bacterial pathogens, such as Bordetella bronchiseptica, Actinobacillus pleuropneumoniae, Glaesserella (Haemophilus) parasuis, and Pasteurella multocida, breach this barrier to lead to local or systematic infections. Here, we demonstrated that respiratory bacterial pathogen infection disrupted the airway epithelial intercellular junction protein, E-cadherin, thus contributing to impaired epithelial cell integrity. E-cadherin knocking-out in newborn pig tracheal cells via CRISPR/Cas9 editing technology confirmed that E-cadherin was sufficient to suppress the paracellular transmigration of these porcine respiratory bacterial pathogens, including G. parasuis, A. pleuropneumoniae, P. multocida, and B. bronchiseptica. The E-cadherin ectodomain cleavage by these pathogens was probably attributed to bacterial HtrA/DegQ protease, but not host HtrA1, MMP7 and ADAM10, and the prominent proteolytic activity was further confirmed by a serine-to-alanine substitution mutation in the active center of HtrA/DegQ protein. Moreover, deletion of the htrA gene in G. parasuis led to severe defects in E-cadherin ectodomain cleavage, cell adherence and paracellular transmigration in vitro, as well as bacterial breaking through the tracheal epithelial cells, systemic invasion and dissemination in vivo. This common pathogenic mechanism shared by other porcine respiratory bacterial pathogens explains how these bacterial pathogens destroy the airway epithelial cell barriers and proliferate in respiratory mucosal surface or other systemic tissues.
Keywords: Airway epithelial barrier; E-cadherin; Glaesserella parasuis; HtrA; paracellular transmigration; porcine respiratory disease complex.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Drolia R, Bhunia AK.. Crossing the intestinal barrier via listeria adhesion protein and internalin A. Trends Microbiol. 2019;27(5):408–425. - PubMed
-
- Wang Q, Margolis B. Apical junctional complexes and cell polarity. Kidney Int. 2007;72(12):1448–1458. - PubMed
-
- Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008;20(2):227–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
