Rejuvenation of Helicobacter pylori-Associated Atrophic Gastritis Through Concerted Actions of Placenta-Derived Mesenchymal Stem Cells Prevented Gastric Cancer
- PMID: 34483897
- PMCID: PMC8416416
- DOI: 10.3389/fphar.2021.675443
Rejuvenation of Helicobacter pylori-Associated Atrophic Gastritis Through Concerted Actions of Placenta-Derived Mesenchymal Stem Cells Prevented Gastric Cancer
Abstract
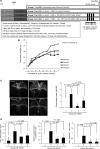
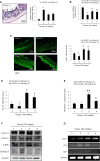
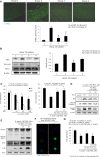
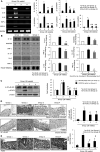
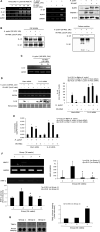
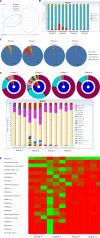
Chronic Helicobacter pylori infection causes gastric cancer via the progression of precancerous chronic atrophic gastritis (CAG). Therefore, repairing gastric atrophy could be a useful strategy in preventing H. pylori-associated gastric carcinogenesis. Although eradication of the bacterial pathogen offers one solution to this association, this study was designed to evaluate an alternative approach using mesenchymal stem cells to treat CAG and prevent carcinogenesis. Here, we used human placenta-derived mesenchymal stem cells (PD-MSCs) and their conditioned medium (CM) to treat H. pylori-associated CAG in a mice/cell model to explore their therapeutic effects and elucidate their molecular mechanisms. We compared the changes in the fecal microbiomes in response to PD-MSC treatments, and chronic H. pylori-infected mice were given ten treatments with PD-MSCs before being sacrificed for end point assays at around 36 weeks of age. These animals presented with significant reductions in the mean body weights of the control group, which were eradicated following PD-MSC treatment (p < 0.01). Significant changes in various pathological parameters including inflammation, gastric atrophy, erosions/ulcers, and dysplastic changes were noted in the control group (p < 0.01), but these were all significantly reduced in the PD-MSC/CM-treated groups. Lgr5+, Ki-67, H+/K+-ATPase, and Musashi-1 expressions were all significantly increased in the treated animals, while inflammatory mediators, MMP, and apoptotic executors were significantly decreased in the PD-MSC group compared to the control group (p < 0.001). Our model showed that H. pylori-initiated, high-salt diet-promoted gastric atrophic gastritis resulted in significant changes in the fecal microbiome at the phylum/genus level and that PD-MSC/CM interventions facilitated a return to more normal microbial communities. In conclusion, administration of PD-MSCs or their conditioned medium may present a novel rejuvenating agent in preventing the progression of H. pylori-associated premalignant lesions.
Keywords: H pylori; cell therapy; chronic atrophic gastritis; inflammation; placenta-derived mesenchymal stem cell; rejuvenation.
Copyright © 2021 Park, Han and Hahm.
Conflict of interest statement
The author KH was employed by the company Medpacto, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

