Ivermectin has New Application in Inhibiting Colorectal Cancer Cell Growth
- PMID: 34483925
- PMCID: PMC8415024
- DOI: 10.3389/fphar.2021.717529
Ivermectin has New Application in Inhibiting Colorectal Cancer Cell Growth
Abstract
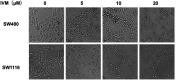
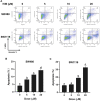
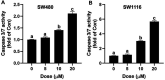
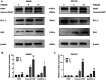
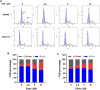
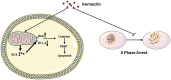
Colorectal cancer (CRC) is the third most common cancer worldwide and still lacks effective therapy. Ivermectin, an antiparasitic drug, has been shown to possess anti-inflammation, anti-virus, and antitumor properties. However, whether ivermectin affects CRC is still unclear. The objective of this study was to evaluate the influence of ivermectin on CRC using CRC cell lines SW480 and SW1116. We used CCK-8 assay to determine the cell viability, used an optical microscope to measure cell morphology, used Annexin V-FITC/7-AAD kit to determine cell apoptosis, used Caspase 3/7 Activity Apoptosis Assay Kit to evaluate Caspase 3/7 activity, used Western blot to determine apoptosis-associated protein expression, and used flow cytometry and fluorescence microscope to determine the reactive oxygen species (ROS) levels and cell cycle. The results demonstrated that ivermectin dose-dependently inhibited colorectal cancer SW480 and SW1116 cell growth, followed by promoting cell apoptosis and increasing Caspase-3/7 activity. Besides, ivermectin upregulated the expression of proapoptotic proteins Bax and cleaved PARP and downregulated antiapoptotic protein Bcl-2. Mechanism analysis showed that ivermectin promoted both total and mitochondrial ROS production in a dose-dependent manner, which could be eliminated by administering N-acetyl-l-cysteine (NAC) in CRC cells. Following NAC treatment, the inhibition of cell growth induced by ivermectin was reversed. Finally, ivermectin at low doses (2.5 and 5 µM) induced CRC cell arrest. Overall, ivermectin suppressed cell proliferation by promoting ROS-mediated mitochondrial apoptosis pathway and inducing S phase arrest in CRC cells, suggesting that ivermectin might be a new potential anticancer drug therapy for human colorectal cancer and other cancers.
Keywords: apoptosis; cell cycle; colorectal cancer; ivermectin; oxidative stress.
Copyright © 2021 Zhou, Wu, Ning, Wu, Xu, Ma, Li, Hu, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

