Low-Density Granulocyte Contamination From Peripheral Blood Mononuclear Cells of Patients With Sepsis and How to Remove It - A Technical Report
- PMID: 34484182
- PMCID: PMC8416421
- DOI: 10.3389/fimmu.2021.684119
Low-Density Granulocyte Contamination From Peripheral Blood Mononuclear Cells of Patients With Sepsis and How to Remove It - A Technical Report
Abstract
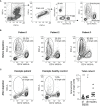
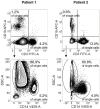
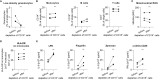
Elucidating the mechanisms contributing to the dysregulated host response to infection as part of the syndrome is a current challenge in sepsis research. Peripheral blood mononuclear cells are widely used in immunological studies. Density gradient centrifugation, a common method, is of limited use for blood drawn from patients with sepsis. A significant number of low-density granulocytes co-purify contributing to low purity of isolated peripheral blood mononuclear cells. Whole blood anticoagulated with lithium heparin was drawn from patients with sepsis (n=14) and healthy volunteers (n=11). Immediately after drawing, the plasma fraction was removed and PBMC were isolated from the cellular fraction by density gradient centrifugation. Samples derived from patients with sepsis were subsequently incubated with cluster of differentiation 15 MicroBeads and granulocytes were depleted using magnetic-activated cell sorting. Core cellular functions as antigen presentation and cytokine secretion were analyzed in cells isolated from healthy volunteers (n=3) before and after depletion to confirm consistent functionality. We report here that depleting CD15+ cells after density gradient centrifugation is a feasible way to get rid of the low-density granulocyte contamination. Afterwards, the purity of isolated, functionally intact peripheral blood mononuclear cells is comparable to healthy volunteers. Information on the isolation purity and identification of the containing cell types are necessary for good comparability between different studies. Depletion of CD15+ cells after density gradient centrifugation is an easy but highly efficient way to gain a higher quality and more reliability in studies using peripheral blood mononuclear cells from septic patients without affecting the functionality of the cells.
Keywords: Ficoll; MACS; PBMC (peripheral blood mononuclear cells); Sepsis; cell isolation; centrifugation; density gradient; low-density granulocytes.
Copyright © 2021 Schenz, Obermaier, Uhle, Weigand and Uhle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

