Elimination of SHIV Infected Cells by Combinations of Bispecific HIVxCD3 DART® Molecules
- PMID: 34484212
- PMCID: PMC8415083
- DOI: 10.3389/fimmu.2021.710273
Elimination of SHIV Infected Cells by Combinations of Bispecific HIVxCD3 DART® Molecules
Abstract
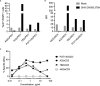
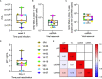
Bispecific HIVxCD3 DART molecules that co-engage the viral envelope glycoprotein (Env) on HIV-1-infected cells and the CD3 receptor on CD3+ T cells are designed to mediate the cytolysis of HIV-1-infected, Env-expressing cells. Using a novel ex vivo system with cells from rhesus macaques (RMs) infected with a chimeric Simian-Human Immunodeficiency Virus (SHIV) CH505 and maintained on ART, we tested the ability of HIVxCD3 DART molecules to mediate elimination of in vitro-reactivated CD4+ T cells in the absence or presence of autologous CD8+ T cells. HIVxCD3 DART molecules with the anti-HIV-1 Env specificities of A32 or 7B2 (non-neutralizing antibodies) or PGT145 (broadly neutralizing antibody) were evaluated individually or combined. DART molecule-mediated antiviral activity increased significantly in the presence of autologous CD8+ T cells. In this ex vivo system, the PGT145 DART molecule was more active than the 7B2 DART molecule, which was more active than the A32 DART molecule. A triple combination of the DART molecules exceeded the activity of the individual PGT145 DART molecule. Modified quantitative virus outgrowth assays confirmed the ability of the DART molecules to redirect RM CD3+ T cells to eliminate SHIV-infected RM CD4+ T cells as demonstrated by the decreased propagation of in vitro infection by the infected cells pre-incubated with DART molecules in presence of effector CD8+ T cells. While mediating cytotoxic activity, DART molecules did not increase proinflammatory cytokine production. In summary, combination of HIVxCD3 DART molecules that have broadly-neutralizing and non-neutralizing anti-HIV-1 Env specificities can leverage the host immune system for treatment of HIV-1 infection but will require appropriate reactivation of the latent reservoir.
Keywords: HIV; bispecific DART molecules; broadly neutralizing antibodies; cytotoxic T cells; non-neutralizing antibodies; redirected cytotoxicity.
Copyright © 2021 Tuyishime, Dashti, Faircloth, Jha, Nordstrom, Haynes, Silvestri, Chahroudi, Margolis and Ferrari.
Conflict of interest statement
JN is employed by MacroGenics and owns MacroGenics stock. JN, BH, and GF have pending patents on some of the molecules. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
SMAC Mimetic Plus Triple-Combination Bispecific HIVxCD3 Retargeting Molecules in SHIV.C.CH505-Infected, Antiretroviral Therapy-Suppressed Rhesus Macaques.J Virol. 2020 Oct 14;94(21):e00793-20. doi: 10.1128/JVI.00793-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32817214 Free PMC article.
-
Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells.J Clin Invest. 2015 Nov 2;125(11):4077-90. doi: 10.1172/JCI82314. Epub 2015 Sep 28. J Clin Invest. 2015. PMID: 26413868 Free PMC article.
-
Redirection of Cord Blood T Cells and Natural Killer Cells for Elimination of Autologous HIV-1-Infected Target Cells Using Bispecific DART® Molecules.Front Immunol. 2020 Apr 21;11:713. doi: 10.3389/fimmu.2020.00713. eCollection 2020. Front Immunol. 2020. PMID: 32373131 Free PMC article.
-
Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies.Transfus Clin Biol. 2001 Aug;8(4):350-8. doi: 10.1016/s1246-7820(01)00187-2. Transfus Clin Biol. 2001. PMID: 11642027 Review.
-
Understanding the basis of CD4(+) T-cell depletion in macaques infected by a simian-human immunodeficiency virus.Vaccine. 2002 May 6;20(15):1934-7. doi: 10.1016/s0264-410x(02)00072-5. Vaccine. 2002. PMID: 11983249 Review.
Cited by
-
Anti-viral efficacy of a next-generation CD4-binding site bNAb in SHIV-infected animals in the absence of anti-drug antibody responses.iScience. 2022 Sep 5;25(10):105067. doi: 10.1016/j.isci.2022.105067. eCollection 2022 Oct 21. iScience. 2022. PMID: 36157588 Free PMC article.
-
Broadly neutralizing antibodies targeting HIV: Progress and challenges.Clin Immunol. 2023 Dec;257:109809. doi: 10.1016/j.clim.2023.109809. Epub 2023 Oct 16. Clin Immunol. 2023. PMID: 37852345 Free PMC article. Review.
-
Bispecific antibody-derived molecules to target persistent HIV infection.J Virus Erad. 2022 Aug 28;8(3):100083. doi: 10.1016/j.jve.2022.100083. eCollection 2022 Sep. J Virus Erad. 2022. PMID: 36111287 Free PMC article.
-
SMAC Mimetic Plus Triple-Combination Bispecific HIVxCD3 Retargeting Molecules in SHIV.C.CH505-Infected, Antiretroviral Therapy-Suppressed Rhesus Macaques.J Virol. 2020 Oct 14;94(21):e00793-20. doi: 10.1128/JVI.00793-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32817214 Free PMC article.
-
Bridging the gap with multispecific immune cell engagers in cancer and infectious diseases.Cell Mol Immunol. 2024 Jul;21(7):643-661. doi: 10.1038/s41423-024-01176-4. Epub 2024 May 24. Cell Mol Immunol. 2024. PMID: 38789528 Free PMC article. Review.
References
-
- Cohen MS. “Successful Treatment of HIV Eliminates Sexual Transmission”. In: Lancet. England: London; (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

