Regulation of Laminaria Polysaccharides with Different Degrees of Sulfation during the Growth of Calcium Oxalate Crystals and their Protective Effects on Renal Epithelial Cells
- PMID: 34484564
- PMCID: PMC8413062
- DOI: 10.1155/2021/5555796
Regulation of Laminaria Polysaccharides with Different Degrees of Sulfation during the Growth of Calcium Oxalate Crystals and their Protective Effects on Renal Epithelial Cells
Abstract
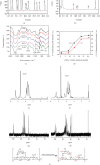
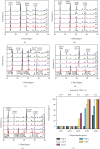
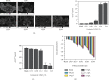
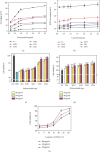
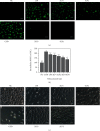
The original Laminaria polysaccharide (LP0) was sulfated using the sulfur trioxide-pyridine method, and four sulfated Laminaria polysaccharides (SLPs) were obtained, namely, SLP1, SLP2, SLP3, and SLP4. The sulfated (-OSO3 -) contents were 8.58%, 15.1%, 22.8%, and 31.3%, respectively. The structures of the polysaccharides were characterized using a Fourier transform infrared (FT-IR) spectrometer and nuclear magnetic resonance (NMR) techniques. SLPs showed better antioxidant activity than LP0, increased the concentration of soluble Ca2+ in the solution, reduced the amount of CaOx precipitation and degree of CaOx crystal aggregation, induced COD crystal formation, and protected HK-2 cells from damage caused by nanometer calcium oxalate crystals. These effects can inhibit the formation of CaOx kidney stones. The biological activity of the polysaccharides increased with the content of -OSO3 -, that is, the biological activities of the polysaccharides had the following order: LP0 < SLP1 < SLP2 < SLP3 < SLP4. These results reveal that SLPs with high -OSO3 - contents are potential drugs for effectively inhibiting the formation of CaOx stones.
Copyright © 2021 Wei-Bo Huang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Khamchun S., Sueksakit K., Chaiyarit S., Thongboonkerd V. Modulatory effects of fibronectin on calcium oxalate crystallization, growth, aggregation, adhesion on renal tubular cells, and invasion through extracellular matrix. Journal of Biological Inorganic Chemistry. 2019;24(2):235–246. doi: 10.1007/s00775-019-01641-w. - DOI - PubMed
-
- Mirković M., Dosen A., Erić S., Vulić P., Matović B., Rosić A. Phase and microstructural study of urinary stones. Microchemical Journal. 2020;152 doi: 10.1016/j.microc.2019.104429. - DOI
-
- Parvaneh L. S., Donadio D., Sulpizi M. Molecular mechanism of crystal growth inhibition at the calcium oxalate/water interfaces. The Journal of Physical Chemistry C. 2016;120(8):4410–4417. doi: 10.1021/acs.jpcc.5b12474. - DOI
-
- de Bellis R., Piacentini M. P., Meli M. A., et al. In vitro effects on calcium oxalate crystallization kinetics and crystal morphology of an aqueous extract from ceterach officinarum: analysis of a potential antilithiatic mechanism. PLoS One. 2019;14(6, article e0218734) doi: 10.1371/journal.pone.0218734. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

