Functional group introduction and aromatic unit variation in a set of π-conjugated macrocycles: revealing the central role of local and global aromaticity
- PMID: 34484800
- PMCID: PMC8382046
- DOI: 10.1039/d1qo00901j
Functional group introduction and aromatic unit variation in a set of π-conjugated macrocycles: revealing the central role of local and global aromaticity
Abstract
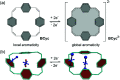
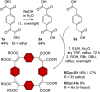
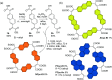
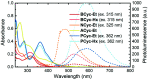
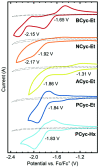
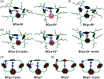
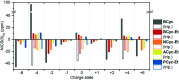
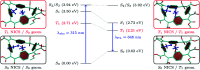
π-Conjugated macrocycles are molecules with unique properties that are increasingly exploited for applications and the question of whether they can sustain global aromatic or antiaromatic ring currents is particularly intriguing. However, there are only a small number of experimental studies that investigate how the properties of π-conjugated macrocycles evolve with systematic structural changes. Here, we present such a systematic experimental study of a set of [2.2.2.2]cyclophanetetraenes, all with formally Hückel antiaromatic ground states, and combine it with an in-depth computational analysis. The study reveals the central role of local and global aromaticity for rationalizing the observed optoelectronic properties, ranging from extremely large Stokes shifts of up to 1.6 eV to reversible fourfold reduction, a highly useful feature for charge storage/accumulation applications. A recently developed method for the visualization of chemical shielding tensors (VIST) is applied to provide unique insight into local and global ring currents occurring in different planes along the macrocycle. Conformational changes as a result of the structural variations can further explain some of the observations. The study contributes to the development of structure-property relationships and molecular design guidelines and will help to understand, rationalize, and predict the properties of other π-conjugated macrocycles. It will also assist in the design of macrocycle-based supramolecular elements with defined properties.
This journal is © the Partner Organisations.
Conflict of interest statement
There are no conflicts to declare.
Figures



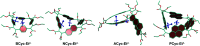
References
-
- Ball M. Zhong Y. Fowler B. Zhang B. Li P. Etkin G. Paley D. W. Decatur J. Dalsania A. K. Li H. Xiao S. Ng F. Steigerwald M. L. Nuckolls C. Macrocyclization in the Design of Organic n-Type Electronic Materials. J. Am. Chem. Soc. 2016;138(39):12861–12867. doi: 10.1021/jacs.6b05474. doi: 10.1021/jacs.6b05474. - DOI - PubMed
-
- Zhang B. Hernández Sánchez R. Zhong Y. Ball M. Terban M. W. Paley D. Billinge S. J. L. Ng F. Steigerwald M. L. Nuckolls C. Hollow organic capsules assemble into cellular semiconductors. Nat. Commun. 2018;9(1):1957. doi: 10.1038/s41467-018-04246-0. doi: 10.1038/s41467-018-04246-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
