Human Neural Stem Cells Flown into Space Proliferate and Generate Young Neurons
- PMID: 34484810
- PMCID: PMC8412175
- DOI: 10.3390/app9194042
Human Neural Stem Cells Flown into Space Proliferate and Generate Young Neurons
Abstract
Here we demonstrate that human neural stem cells (NSCs) proliferate while in space and they express specific NSC markers after being in space. NSCs displayed both higher oxygen consumption and glycolysis than ground controls. These cells also kept their ability to become young neurons. Electrophysiological recordings of space NSC-derived neurons showed immature cell membrane properties characterized by small capacitance and very high input resistance. Current injections elicited only an incipient action potential. No spontaneous synaptic events could be detected, suggesting their immature status even though most recorded cells displayed complex morphology and numerous cell processes. Ascertaining the origin of the NSCs' increased energy requirement is of the essence in order to design effective measures to minimize health risks associated with long-duration human spaceflight missions.
Keywords: energetics; glycolysis; microgravity; neural stem cells; neuronal specification; neurons; pluripotency; proliferation; space flight.
Conflict of interest statement
Conflicts of Interest: The authors declare that they have no conflict of interest.
Figures


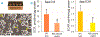


References
-
- Lee AG; Mader TH; Gibson CR; Tarver W Space Flight-Associated Neuro-ocular Syndrome. JAMA Ophthalmol. 2017,135,992–994. - PubMed
-
- Studer M; Bradacs G; Hilliger A; Hürlimann E; Engeli S; Thiel CS; Zeitner P; Denier B; Binggeli M; Syburra T; et al. Parabolic maneuvers of the Swiss Air Force fighter jet F-5E as a research platform for cell culture experiments in microgravity. Acta Astronaut. 2011, 68,1729–1741.
-
- Silvano M; Miele E; Valerio MC; Casadei L; Begalli F; Campese AF; Besharat ZM; Alfano V; Abballe L; Catanzaro G; et al. Consequences of Simulated Microgravity in Neural Stem Cells: Biological Effects and Metabolic Response. J. Stem Cell Res. Ther 2015, 5, 289.
