Avelumab internalization by human circulating immune cells is mediated by both Fc gamma receptor and PD-L1 binding
- PMID: 34484871
- PMCID: PMC8409756
- DOI: 10.1080/2162402X.2021.1958590
Avelumab internalization by human circulating immune cells is mediated by both Fc gamma receptor and PD-L1 binding
Abstract
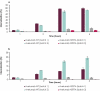
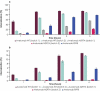
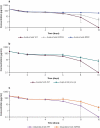
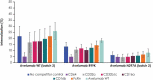
Avelumab is an IgG1 anti-programmed death ligand 1 (anti-PD-L1) monoclonal antibody that has been approved as a monotherapy for metastatic Merkel cell carcinoma and advanced urothelial carcinoma, and in combination with axitinib for advanced renal cell carcinoma. Avelumab is cleared faster and has a shorter half-life than other anti-PD-L1 antibodies, such as atezolizumab and durvalumab, but the mechanisms underlying these differences are unknown. IgG antibodies can be cleared through receptor-mediated endocytosis after binding of the antibody Fab region to target proteins, or via Fcγ receptor (FcγR)-mediated endocytosis. Unlike other approved anti-PD-L1 antibodies, avelumab has a native Fc region that retains FcγR binding capability. We hypothesized that the rapid clearance of avelumab might be due to the synergistic effect of both FcγR-mediated and PD-L1 target-mediated internalization. To investigate this, we performed in vitro and in vivo studies that compared engineered variants of avelumab and atezolizumab to determine mechanisms of cellular internalization. We found that both FcγR and PD-L1 binding contribute to avelumab internalization. While FcγR binding was the dominant mechanism of avelumab internalization in vitro, with CD64 acting as the most important FcγR, studies in mice and cynomolgus monkeys showed that both FcγR and PD-L1 contribute to avelumab elimination, with PD-L1 binding playing a greater role. These studies suggest that the rapid internalization of avelumab might be due to simultaneous binding of both PD-L1 and FcγR in trans. Our findings also provide a basis to alter the clearance and half-life of monoclonal antibodies in therapeutic development.
Keywords: Avelumab; FCγr binding; PD-L1 binding; clearance; internalization.
© 2021 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures


References
-
- Hamilton G, Rath B. Avelumab: combining immune checkpoint inhibition and antibody-dependent cytotoxicity. Expert Opin Biol Ther. 2017;17:515–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
