Fampridine and Acetazolamide in EA2 and Related Familial EA: A Prospective Randomized Placebo-Controlled Trial
- PMID: 34484942
- PMCID: PMC8382428
- DOI: 10.1212/CPJ.0000000000001017
Fampridine and Acetazolamide in EA2 and Related Familial EA: A Prospective Randomized Placebo-Controlled Trial
Abstract
Objective: To determine the efficacy and safety of the treatment with prolonged-release 4-aminopyridine (fampridine) and acetazolamide for patients with episodic ataxia type 2 (EA2), patients with EA2 were treated with a random sequence of fampridine, acetazolamide, and placebo in a 3-period crossover trial.
Methods: A total of 30 patients with EA2 (8 female; aged 20-71 years; 18 genetically confirmed, 4 with a positive family history, 8 with the clinical diagnosis) were enrolled in this phase III, randomized, double-blind, placebo-controlled, 3-period crossover trial. Each period lasted 12 weeks with a 4-week washout period. Each patient received a random sequence of 20 mg/d fampridine, 750 mg/d acetazolamide, and placebo. The primary end point was the number of attacks during the last 30 days within the 12-week treatment period. Participants, caregivers, and those assessing the outcomes were blinded to the intervention.
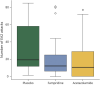
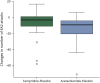
Results: Compared with placebo, fampridine reduced the number of attacks to 63% (95% CI 54%-74%) and acetazolamide to 52% (95% CI 46%-60%). A total of 39 (26.5%) adverse events were observed under treatment with fampridine (mostly tingling paresthesia and fatigue), 66 (44.9%) happened under acetazolamide (mostly taste disturbance and gastrointestinal complaints), and 42 (28.6%) under placebo (mostly gastrointestinal complaints).
Conclusion: Both fampridine and acetazolamide significantly reduce the number of attacks in patients with EA2 and related EA in comparison to placebo. Fampridine 10 mg twice daily had fewer side effects than acetazolamide 250 mg 3 times daily. The trial was registered with DRKS.de (DRKS00005258) and EudraCT (2013-000107-17). This study was supported by the Federal Ministry of Education and Research (BMBF) (grant number 01EO0901). Fampridine (study medication) was provided by Biogen Idec.
Classification of evidence: Class II evidence.
© 2021 American Academy of Neurology.
Figures

References
-
- Jen JC, Graves TD, Hess EJ, Hanna MG, Griggs RC, Baloh RW. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain 2007;130:2484–2493. - PubMed
-
- Jen JC, Wan J. Episodic ataxias. Handb Clin Neurol 2018;155:205–215. - PubMed
-
- Jen J, Kim GW, Baloh RW. Clinical spectrum of episodic ataxia type 2. Neurology 2004;62:17–22. - PubMed
-
- Denier C, Ducros A, Vahedi K, et al. High prevalence of CACNA1A truncations and broader clinical spectrum in episodic ataxia type 2. Neurology 1999;52:1816. - PubMed
-
- Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca 2 channel gene CACNL1A4. Cell 1996;87:543–552. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
