Microbial Warfare on Three Fronts: Mixed Biofilm of Aspergillus fumigatus and Staphylococcus aureus on Primary Cultures of Human Limbo-Corneal Fibroblasts
- PMID: 34485167
- PMCID: PMC8415486
- DOI: 10.3389/fcimb.2021.646054
Microbial Warfare on Three Fronts: Mixed Biofilm of Aspergillus fumigatus and Staphylococcus aureus on Primary Cultures of Human Limbo-Corneal Fibroblasts
Abstract
Background: Coinfections with fungi and bacteria in ocular pathologies are increasing at an alarming rate. Two of the main etiologic agents of infections on the corneal surface, such as Aspergillus fumigatus and Staphylococcus aureus, can form a biofilm. However, mixed fungal-bacterial biofilms are rarely reported in ocular infections. The implementation of cell cultures as a study model related to biofilm microbial keratitis will allow understanding the pathogenesis in the cornea. The cornea maintains a pathogen-free ocular surface in which human limbo-corneal fibroblast cells are part of its cell regeneration process. There are no reports of biofilm formation assays on limbo-corneal fibroblasts, as well as their behavior with a polymicrobial infection.
Objective: To determine the capacity of biofilm formation during this fungal-bacterial interaction on primary limbo-corneal fibroblast monolayers.
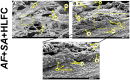
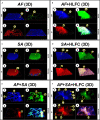
Results: The biofilm on the limbo-corneal fibroblast culture was analyzed by assessing biomass production and determining metabolic activity. Furthermore, the mixed biofilm effect on this cell culture was observed with several microscopy techniques. The single and mixed biofilm was higher on the limbo-corneal fibroblast monolayer than on abiotic surfaces. The A. fumigatus biofilm on the human limbo-corneal fibroblast culture showed a considerable decrease compared to the S. aureus biofilm on the limbo-corneal fibroblast monolayer. Moreover, the mixed biofilm had a lower density than that of the single biofilm. Antibiosis between A. fumigatus and S. aureus persisted during the challenge to limbo-corneal fibroblasts, but it seems that the fungus was more effectively inhibited.
Conclusion: This is the first report of mixed fungal-bacterial biofilm production and morphological characterization on the limbo-corneal fibroblast monolayer. Three antibiosis behaviors were observed between fungi, bacteria, and limbo-corneal fibroblasts. The mycophagy effect over A. fumigatus by S. aureus was exacerbated on the limbo-corneal fibroblast monolayer. During fungal-bacterial interactions, it appears that limbo-corneal fibroblasts showed some phagocytic activity, demonstrating tripartite relationships during coinfection.
Keywords: Aspergillus fumigatus; Staphylococcus aureus; fungal–bacterial interaction; human limbo-corneal fibroblast cells; mixed fungal–bacterial biofilms.
Copyright © 2021 Ramírez-Granillo, Bautista-Hernández, Bautista-De Lucío, Magaña-Guerrero, Domínguez-López, Córdova-Alcántara, Pérez, Martínez-Rivera and Rodríguez-Tovar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Abelson M. B., McLaughlin J. (2012). Of Biomes, Biofilm and the Ocular Surface. Rev. Ophthalmol. 19 (9), 52.
-
- Álvarez-Félix J. R., Palazuelos-Gaxiola M., López-López G. (2010). Prevalencia De Queratitis En El Servicio De Oftalmología De La Coordinación Universitaria Del Hospital Civil De Culiacán. Rev. Med. UAS Nueva época 1 (3), 11–14.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

