Branched-Chain Amino Acid Catabolism and Cardiopulmonary Function Following Acute Maximal Exercise Testing in Adolescents
- PMID: 34485418
- PMCID: PMC8416443
- DOI: 10.3389/fcvm.2021.721354
Branched-Chain Amino Acid Catabolism and Cardiopulmonary Function Following Acute Maximal Exercise Testing in Adolescents
Abstract
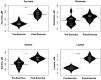
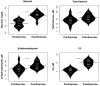
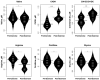
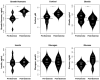
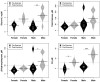
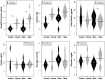
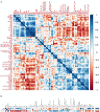
Background: To provide energy for cardiopulmonary function and maintenance of blood glucose, acute aerobic exercise induces lipolysis, fatty acid oxidation (FAO), glycolysis, and glycogenolysis/gluconeogenesis. These adaptations are mediated by increases in cortisol, growth hormone (GH), and catecholamines and facilitated by a decline in insulin. Branched-chain amino acids (BCAA) also undergo catabolism during intense exercise. Here, we investigated the relationship between BCAA catabolism and metrics of cardiopulmonary function in healthy, well-developed, mature adolescent athletes undergoing an acute bout of maximal aerobic exercise. Hypothesis: We hypothesized: (a) acute maximal exercise in adolescents induces lipolysis, FAO, and BCAA catabolism associated with increases in GH and cortisol and a reduction in insulin; (b) increases in GH are associated with increases in ghrelin; and (c) metrics of cardiopulmonary function (aVO2, rVO2, aVO2/HRmax) following maximal exercise correlate with increases in GH secretion, FAO, and BCAA catabolism. Methods: Blood samples before and after maximal cardiopulmonary exercise in 11 adolescent athletes were analyzed by tandem-mass spectrometry. Paired, two-tailed student's t-tests identified significant changes following exercise. Linear regression determined if pre-exercise metabolite levels, or changes in metabolite levels, were associated with aVO2, rVO2, and aVO2/HRmax. Sex and school of origin were included as covariates in all regression analyses. Results: Following exercise there were increases in GH and cortisol, and decreases in ghrelin, but no changes in glucose or insulin concentrations. Suggesting increased lipolysis and FAO, the levels of glycerol, ketones, β-hydroxybutyrate, and acetylcarnitine concentrations increased. Pyruvate, lactate, alanine, and glutamate concentrations also increased. Plasma concentrations of valine (a BCAA) declined (p = 0.002) while valine degradation byproducts increased in association with decreases in urea cycle amino acids arginine and ornithine. Metrics of cardiopulmonary function were associated with increases in propionylcarnitine (C3, p = 0.013) and Ci4-DC/C4-DC (p < 0.01), byproducts of BCAA catabolism. Conclusions: Induction of lipolysis, FAO, gluconeogenesis, and glycogenolysis provides critical substrates for cardiopulmonary function during exercise. However, none of those pathways were significantly associated with metrics of cardiopulmonary function. The associations between rVO2, and aVO2/HRmax and C3 and Ci4-DC/C4-DC suggest that the cardiopulmonary response to maximal exercise in adolescents is linked to BCAA utilization and catabolism.
Keywords: BCAA; ghrelin; growth hormone; rVO2; total body oxygen consumption (VO2).
Copyright © 2021 Gumus Balikcioglu, Ramaker, Mason, Huffman, Johnson, Ilkayeva, Muehlbauer, Freemark and Kraus.
Conflict of interest statement
PGB receives support from Diabetes Research Connection, but has no relevant disclosures to this manuscript. MF is a co-investigator on a grant from the American Heart Association that deals with the pathogenesis and treatment of childhood obesity. MF is also the local PI on a Rhythm-sponsored study of identification and treatment of children and adults with monogenic obesity, and member of a Data Safety Monitoring Board for a separate Rhythm-sponsored study of treatment of patients with syndromic obesity. KM receives support from the Cystic Fibrosis Foundation, but has no relevant disclosures to this manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- U.S. Department of Health and Human Services . Physical Activity and Health: A Report of the Surgeon General. Ch3 Physiologic Responses and Long-Term Adaptations to Exercise. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; (1996).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

