Reproducible immortalization of erythroblasts from multiple stem cell sources provides approach for sustainable RBC therapeutics
- PMID: 34485592
- PMCID: PMC8390520
- DOI: 10.1016/j.omtm.2021.06.002
Reproducible immortalization of erythroblasts from multiple stem cell sources provides approach for sustainable RBC therapeutics
Abstract
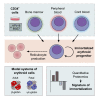
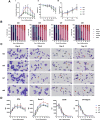
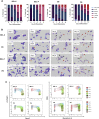
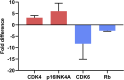
Developing robust methodology for the sustainable production of red blood cells in vitro is essential for providing an alternative source of clinical-quality blood, particularly for individuals with rare blood group phenotypes. Immortalized erythroid progenitor cell lines are the most promising emergent technology for achieving this goal. We previously created the erythroid cell line BEL-A from bone marrow CD34+ cells that had improved differentiation and enucleation potential compared to other lines reported. In this study we show that our immortalization approach is reproducible for erythroid cells differentiated from bone marrow and also from far more accessible peripheral and cord blood CD34+ cells, consistently generating lines with similar improved erythroid performance. Extensive characterization of the lines shows them to accurately recapitulate their primary cell equivalents and provides a molecular signature for immortalization. In addition, we show that only cells at a specific stage of erythropoiesis, predominantly proerythroblasts, are amenable to immortalization. Our methodology provides a step forward in the drive for a sustainable supply of red cells for clinical use and for the generation of model cellular systems for the study of erythropoiesis in health and disease, with the added benefit of an indefinite expansion window for manipulation of molecular targets.
Keywords: bone marrow; cell lines; cord blood; erythroid; immortalized; peripheral blood; proteomics.
© 2021.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Timmins N.E., Athanasas S., Günther M., Buntine P., Nielsen L.K. Ultra-high-yield manufacture of red blood cells from hematopoietic stem cells. Tissue Eng. Part C Methods. 2011;17:1131–1137. - PubMed
-
- Griffiths R.E., Kupzig S., Cogan N., Mankelow T.J., Betin V.M.S., Trakarnsanga K., Massey E.J., Lane J.D., Parsons S.F., Anstee D.J. Maturing reticulocytes internalize plasma membrane in glycophorin A-containing vesicles that fuse with autophagosomes before exocytosis. Blood. 2012;119:6296–6306. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

