Effects of Selenium treatment on cardiac function in Chagas heart disease: Results from the STCC randomized Trial
- PMID: 34485877
- PMCID: PMC8406152
- DOI: 10.1016/j.eclinm.2021.101105
Effects of Selenium treatment on cardiac function in Chagas heart disease: Results from the STCC randomized Trial
Abstract
Background: Chagas disease (caused by Trypanosoma cruzi infection) evolves to chronic chagasic cardiomyopathy (CCC) affecting 1.8 million people worldwide. This is the first randomized, placebo-controlled, double-blinded, clinical trial designed to estimate efficacy and safety of selenium (Se) treatment in CCC.
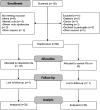
Methods: 66 patients with CCC stages B1 (left ventricular ejection fraction [LVEF] > 45% and no heart failure; n = 54) or B2 (LVEF < 45% and no heart failure; n = 12) were randomly assigned to receive 100 mcg/day sodium selenite (Se, n = 32) or placebo (Pla, n = 34) for one year (study period: May 2014-September 2018). LVEF changes over time and adverse effects were investigated. Trial registration number: NCT00875173 (clinicaltrials.gov).
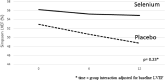
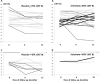
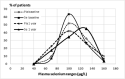
Findings: No significant differences between the two groups were observed for the primary outcome: mean LVEF after 6 (β= +1.1 p = 0.51 for Se vs Pla) and 12 months (β= +2.1; p = 0.23). In a subgroup analysis, statistically significant longitudinal changes were observed for mean LVEF in the stage B2 subgroup (β= +10.1; p = 0.02 for Se [n = 4] vs Pla [n = 8]). Se treatment was safe for CCC patients, and the few adverse effects observed were similarly distributed across the two groups.
Interpretation: Se treatment did not improve cardiac function (evaluated from LVEF) in CCC. However, in the subgroup of patients at B2 stage, a potential beneficial influence of Se was observed. Complementary studies are necessary to explore diverse Se dose and/or associations in different CCC stages (B2 and C), as well as in A and B1 stages with longer follow-up.
Funding: Brazilian Ministry of Health, Fiocruz, CNPq, FAPERJ.
© 2021 The Authors.
Conflict of interest statement
The authors have nothing to disclose.
Figures
References
-
- World Health Organization. Chagas disease (American trypanosomiasis). Fact sheet updated March 11th, 2020. Last accessed on March 07 2021,, https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(america...)
-
- Pérez-Molina J.A., Molina I. Chagas disease. Lancet. 2017;(17):31612–31614. doi: 10.1016/S0140-6736(17)31612-4. S0140-6736. - DOI
Associated data
LinkOut - more resources
Full Text Sources
Medical

