International study on the outcome of locoregional therapy for liver transplant in hepatocellular carcinoma beyond Milan criteria
- PMID: 34485882
- PMCID: PMC8405981
- DOI: 10.1016/j.jhepr.2021.100331
International study on the outcome of locoregional therapy for liver transplant in hepatocellular carcinoma beyond Milan criteria
Abstract
Background & aims: Good outcomes after liver transplantation (LT) have been reported after successfully downstaging to Milan criteria in more advanced hepatocellular carcinoma (HCC). We aimed to compare post-LT outcomes in patients receiving locoregional therapies (LRT) before LT according to Milan criteria and University of California San Francisco downstaging (UCSF-DS) protocol and 'all-comers'.
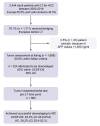
Methods: This multicentre cohort study included patients who received any LRT before LT from Europe and Latin America (2000-2018). We excluded patients with alpha-foetoprotein (AFP) above 1,000 ng/ml. Competing risk regression analysis for HCC recurrence was conducted, estimating subdistribution hazard ratios (SHRs) and corresponding 95% CIs.
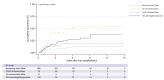
Results: From 2,441 LT patients, 70.1% received LRT before LT (n = 1,711). Of these, 80.6% were within Milan, 12.0% within UCSF-DS, and 7.4% all-comers. Successful downstaging was achieved in 45.2% (CI 34.8-55.8) and 38.2% (CI 25.4-52.3) of the UCSF-DS group and all-comers, respectively. The risk of recurrence was higher for all-comers (SHR 6.01 [p <0.0001]) and not significantly higher for the UCSF-DS group (SHR 1.60 [p = 0.32]), compared with patients remaining within Milan. The all-comers presented more frequent features of aggressive HCC and higher tumour burden at explant. Among the UCSF-DS group, an AFP value of ≤20 ng/ml at listing was associated with lower recurrence (SHR 2.01 [p = 0.006]) and better survival. However, recurrence was still significantly high irrespective of AFP ≤20 ng/ml in all-comers.
Conclusions: Patients within the UCSF-DS protocol at listing have similar post-transplant outcomes compared with those within Milan when successfully downstaged. Meanwhile, all-comers have a higher recurrence and inferior survival irrespective of response to LRT. Additionally, in the UCSF-DS group, an ALP of ≤20 ng/ml might be a novel tool to optimise selection of candidates for LT.
Clinical trial number: This study was registered as part of an open public registry (NCT03775863).
Lay summary: Patients with more extended HCC (within the UCSF-DS protocol) successfully downstaged to the conventional Milan criteria do not have a higher recurrence rate after LT compared with the group remaining in the Milan criteria from listing to transplantation. Moreover, in the UCSF-DS patient group, an ALP value equal to or below 20 ng/ml at listing might be a novel tool to further optimise selection of candidates for LT.
Keywords: AC, all-comers; AFP, alpha-foetoprotein; All-comers; Alpha-foetoprotein; DS, downstaging; Downstaging; EASL, European Association for the Study of the Liver; HCC, hepatocellular carcinoma; HR, hazard ratio; Hepatocellular carcinoma; ITT, intention to treat; LR, liver resection; LRT, locoregional therapies; LT, liver transplantation; MC, Milan criteria; MVI, microvascular invasion; PEI, percutaneous ethanol ablation; RFA, radiofrequency ablation; SHR, subdistribution hazard ratio; TACE, transarterial chemoembolisation; UCSF downstaging protocol; UCSF-DS, University of California San Francisco downstaging; UNOS, United Network for Organ Sharing; WL, waiting list.
© 2021 The Authors.
Conflict of interest statement
There are no conflicts of interest to declare. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Mazzaferro V., Regalia E., Doci R., Andreola S., Pulvirenti A., Bozzetti F. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. - PubMed
-
- Degroote H., Callebout E., Iesari S., Dekervel J., Schreiber J., Pirenne J. Extended criteria for liver transplantation in hepatocellular carcinoma. A retrospective, multicentric validation study in Belgium. Surg Oncol. 2020;33:231–238. - PubMed
-
- Yao F.Y., Xiao L., Bass N.M., Kerlan R., Ascher N.L., Roberts J.P. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transpl. 2007;7:2587–2596. - PubMed
-
- Duvoux C., Roudot-Thoraval F., Decaens T., Pessione F., Badran H., Piardi T. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143 986–994 e3. - PubMed
-
- Mazzaferro V., Sposito C., Zhou J., Pinna A.D., De Carlis L., Fan J. Metroticket 2.0 Model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154:128–139. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

