Fab and Fc contribute to maximal protection against SARS-CoV-2 following NVX-CoV2373 subunit vaccine with Matrix-M vaccination
- PMID: 34485950
- PMCID: PMC8405506
- DOI: 10.1016/j.xcrm.2021.100405
Fab and Fc contribute to maximal protection against SARS-CoV-2 following NVX-CoV2373 subunit vaccine with Matrix-M vaccination
Abstract
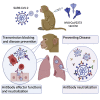
Recently approved vaccines have shown remarkable efficacy in limiting SARS-CoV-2-associated disease. However, with the variety of vaccines, immunization strategies, and waning antibody titers, defining the correlates of immunity across a spectrum of antibody titers is urgently required. Thus, we profiled the humoral immune response in a cohort of non-human primates immunized with a recombinant SARS-CoV-2 spike glycoprotein (NVX-CoV2373) at two doses, administered as a single- or two-dose regimen. Both antigen dose and boosting significantly altered neutralization titers and Fc-effector profiles, driving unique vaccine-induced antibody fingerprints. Combined differences in antibody effector functions and neutralization were associated with distinct levels of protection in the upper and lower respiratory tract. Moreover, NVX-CoV2373 elicited antibodies that functionally targeted emerging SARS-CoV-2 variants. Collectively, the data presented here suggest that a single dose may prevent disease via combined Fc/Fab functions but that two doses may be essential to block further transmission of SARS-CoV-2 and emerging variants.
Keywords: COVID-19; Fc-function; Fc-receptors; SARS-CoV-2; antibodies; correlates; neutralization; prime-boost; vaccination.
© 2021.
Conflict of interest statement
N.P., M.G.-X., J.-H.T., B.Z., S.M., A.M.G., M.J.M., A.D.P., G.G., G.S., and L.E. are current or past employees of Novavax, Inc. and have stock options in the company. G.A. is the founder of SeromYx Systems, Inc. A.L.Z. is a current employee of Moderna, Inc. but conducted this work before employment. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and NDV-based SARS-CoV-2 vaccines, which list F.K. as co-inventor. F.A. is also listed on the serological assay patent application as a co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. F.K. has consulted for Merck and Pfizer (before 2020) and is currently consulting for Pfizer, Seqirus, and Avimex. The Krammer laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. Y.G.-G., R.C., M.J.G., C.A., K.M.P., C.L., D.Y., K.A.B., M.E.M., J.L., D.M., C.M., S.S., F.A., E.O.S, D.L., and M.B.F. declare no competing interest.
Figures





Update of
-
Collaboration between the Fab and Fc contribute to maximal protection against SARS-CoV-2 in nonhuman primates following NVX-CoV2373 subunit vaccine with Matrix-M™ vaccination.bioRxiv [Preprint]. 2021 Feb 5:2021.02.05.429759. doi: 10.1101/2021.02.05.429759. bioRxiv. 2021. Update in: Cell Rep Med. 2021 Sep 21;2(9):100405. doi: 10.1016/j.xcrm.2021.100405. PMID: 33564763 Free PMC article. Updated. Preprint.
-
Collaboration between the Fab and Fc contribute to maximal protection against SARS-CoV-2 following NVX-CoV2373 subunit vaccine with Matrix-M™ vaccination.Res Sq [Preprint]. 2021 Feb 15:rs.3.rs-200342. doi: 10.21203/rs.3.rs-200342/v1. Res Sq. 2021. Update in: Cell Rep Med. 2021 Sep 21;2(9):100405. doi: 10.1016/j.xcrm.2021.100405. PMID: 33619473 Free PMC article. Updated. Preprint.
References
-
- Day M. Covid-19: identifying and isolating asymptomatic people helped eliminate virus in Italian village. BMJ. 2020;368:m1165. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

