Adverse Maternal Environment and Postweaning Western Diet Alter Hepatic CD36 Expression and Methylation Concurrently with Nonalcoholic Fatty Liver Disease in Mouse Offspring
- PMID: 34486661
- PMCID: PMC8485909
- DOI: 10.1093/jn/nxab249
Adverse Maternal Environment and Postweaning Western Diet Alter Hepatic CD36 Expression and Methylation Concurrently with Nonalcoholic Fatty Liver Disease in Mouse Offspring
Abstract
Background: The role of an adverse maternal environment (AME) in conjunction with a postweaning Western diet (WD) in the development of nonalcoholic fatty liver disease (NAFLD) in adult offspring has not been explored. Likewise, the molecular mechanisms associated with AME-induced NAFLD have not been studied. The fatty acid translocase or cluster of differentiation 36 (CD36) has been implicated to play a causal role in the pathogenesis of WD-induced steatosis. However, it is unknown if CD36 plays a role in AME-induced NAFLD.
Objective: This study was designed to evaluate the isolated and additive impact of AME and postweaning WD on the expression and DNA methylation of hepatic Cd36 in association with the development of NAFLD in a novel mouse model.
Methods: AME constituted maternal WD and maternal stress, whereas the control (Con) group had neither. Female C57BL/6J mice were fed a WD [40% fat energy, 29.1% sucrose energy, and 0.15% cholesterol (wt/wt)] 5 wk prior to pregnancy and throughout lactation. Non invasive variable stressors (random frequent cage changing, limited bedding, novel object, etc.) were applied to WD dams during the last third of pregnancy to produce an AME. Con dams consumed the control diet (CD) (10% fat energy, no sucrose or cholesterol) and were not exposed to stress. Male offspring were weaned onto either CD or WD, creating 4 experimental groups: Con-CD, Con-WD, AME-CD, and AME-WD, and evaluated for metabolic and molecular parameters at 120 d of age.
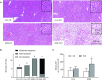
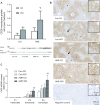
Results: AME and postweaning WD independently and additively increased the development of hepatic steatosis in adult male offspring. AME and WD independently and additively upregulated hepatic CD36 protein and mRNA expression and hypomethylated promoters 2 and 3 of the Cd36 gene.
Conclusions: Using a mouse AME model together with postweaning WD, this study demonstrates a role for CD36 in AME-induced NAFLD in offspring and reveals 2 regions of environmentally induced epigenetic heterogeneity within Cd36.
Keywords: CD36; DNA; NAFLD; maternal environment; methylation; perinatal.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures


References
-
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. - PubMed
-
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–31. - PubMed
-
- Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, Lee JH, Khadem S, Ren S, Li Set al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134(2):556–67.e1. - PubMed
-
- Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Piccolo BD, Mercer KE, Andres A, Thakali KM, Shankar K. Maternal high-fat diet programs offspring liver steatosis in a sexually dimorphic manner in association with changes in gut microbial ecology in mice. Sci Rep. 2018;8(1):16502. - PMC - PubMed

