Complement component 3 from astrocytes mediates retinal ganglion cell loss during neuroinflammation
- PMID: 34487221
- PMCID: PMC8713426
- DOI: 10.1007/s00401-021-02366-4
Complement component 3 from astrocytes mediates retinal ganglion cell loss during neuroinflammation
Abstract
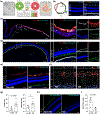
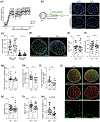
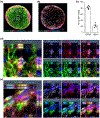
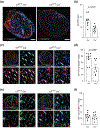
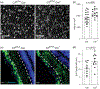
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) characterized by varying degrees of secondary neurodegeneration. Retinal ganglion cells (RGC) are lost in MS in association with optic neuritis but the mechanisms of neuronal injury remain unclear. Complement component C3 has been implicated in retinal and cerebral synaptic pathology that may precede neurodegeneration. Herein, we examined post-mortem MS retinas, and then used a mouse model, experimental autoimmune encephalomyelitis (EAE), to examine the role of C3 in the pathogenesis of RGC loss associated with optic neuritis. First, we show extensive C3 expression in astrocytes (C3+/GFAP+ cells) and significant RGC loss (RBPMS+ cells) in post-mortem retinas from people with MS compared to retinas from non-MS individuals. A patient with progressive MS with a remote history of optic neuritis showed marked reactive astrogliosis with C3 expression in the inner retina extending into deeper layers in the affected eye more than the unaffected eye. To study whether C3 mediates retinal degeneration, we utilized global C3-/- EAE mice and found that they had less RGC loss and partially preserved neurites in the retina compared with C3+/+ EAE mice. C3-/- EAE mice had fewer axonal swellings in the optic nerve, reflecting reduced axonal injury, but had no changes in demyelination or T cell infiltration into the CNS. Using a C3-tdTomato reporter mouse line, we show definitive evidence of C3 expression in astrocytes in the retina and optic nerves of EAE mice. Conditional deletion of C3 in astrocytes showed RGC protection replicating the effects seen in the global knockouts. These data implicate astrocyte C3 expression as a critical mediator of retinal neuronal pathology in EAE and MS, and are consistent with recent studies showing C3 gene variants are associated with faster rates of retinal neurodegeneration in human disease.
Keywords: Astrocyte; Complement component 3; EAE; Multiple sclerosis; Optic nerve; Retina.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Figures


References
-
- Calida DM, Constantinescu C, Purev E, Zhang GX, Ventura ES, Lavi E et al. (2001) Cutting edge: C3, a key component of complement activation, is not required for the development of myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis in mice. J Immunol 166:723–726 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

