Current Concepts on the Pathogenesis of Systemic Sclerosis
- PMID: 34487318
- PMCID: PMC10167130
- DOI: 10.1007/s12016-021-08889-8
Current Concepts on the Pathogenesis of Systemic Sclerosis
Abstract
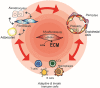
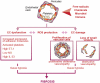
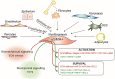
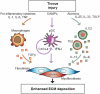
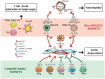
From the clinical standpoint, systemic sclerosis (SSc) is characterized by skin and internal organ fibrosis, diffuse fibroproliferative vascular modifications, and autoimmunity. Clinical presentation and course are highly heterogenous and life expectancy variably affected mostly dependent on lung and heart involvement. SSc touches more women than men with differences in disease severity and environmental exposure. Pathogenetic events originate from altered homeostasis favored by genetic predisposition, environmental cues and a variety of endogenous and exogenous triggers. Epigenetic modifications modulate SSc pathogenesis which strikingly associate profound immune-inflammatory dysregulation, abnormal endothelial cell behavior, and cell trans-differentiation into myofibroblasts. SSc myofibroblasts show enhanced survival and enhanced extracellular matrix deposition presenting altered structure and altered physicochemical properties. Additional cell types of likely pathogenic importance are pericytes, platelets, and keratinocytes in conjunction with their relationship with vessel wall cells and fibroblasts. In SSc, the profibrotic milieu is favored by cell signaling initiated in the one hand by transforming growth factor-beta and related cytokines and in the other hand by innate and adaptive type 2 immune responses. Radical oxygen species and invariant receptors sensing danger participate to altered cell behavior. Conventional and SSc-specific T cell subsets modulate both fibroblasts as well as endothelial cell dysfunction. Beside autoantibodies directed against ubiquitous antigens important for enhanced clinical classification, antigen-specific agonistic autoantibodies may have a pathogenic role. Recent studies based on single-cell RNAseq and multi-omics approaches are revealing unforeseen heterogeneity in SSc cell differentiation and functional states. Advances in system biology applied to the wealth of data generated by unbiased screening are allowing to subgroup patients based on distinct pathogenic mechanisms. Deciphering heterogeneity in pathogenic mechanisms will pave the way to highly needed personalized therapeutic approaches.
Keywords: Fibrosis; Immune responses; Inflammation; Pathogenesis; System biology; Systemic sclerosis.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Elhai M, Meune C, Boubaya M, Avouac J, Hachulla E, Balbir-Gurman A, Riemekasten G, Airo P, Joven B, Vettori S, Cozzi F, Ullman S, Czirjak L, Tikly M, Muller-Ladner U, Caramaschi P, Distler O, Iannone F, Ananieva LP, Hesselstrand R, Becvar R, Gabrielli A, Damjanov N, Salvador MJ, Riccieri V, Mihai C, Szucs G, Walker UA, Hunzelmann N, Martinovic D, Smith V, Muller CS, Montecucco CM, Opris D, Ingegnoli F, Vlachoyiannopoulos PG, Stamenkovic B, Rosato E, Heitmann S, Distler JHW, Zenone T, Seidel M, Vacca A, Langhe E, Novak S, Cutolo M, Mouthon L, Henes J, Chizzolini C, Muhlen CAV, Solanki K, Rednic S, Stamp L, Anic B, Santamaria VO, De Santis M, Yavuz S, Sifuentes-Giraldo WA, Chatelus E, Stork J, Laar JV, Loyo E, de la Pena Garcia, Lefebvre P, Eyerich K, Cosentino V, Alegre-Sancho JJ, Kowal-Bielecka O, Rey G, Matucci-Cerinic M, Allanore Y. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis. 2017;76:1897–1905. doi: 10.1136/annrheumdis-2017-211448. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

