HspB8 prevents aberrant phase transitions of FUS by chaperoning its folded RNA-binding domain
- PMID: 34487489
- PMCID: PMC8510580
- DOI: 10.7554/eLife.69377
HspB8 prevents aberrant phase transitions of FUS by chaperoning its folded RNA-binding domain
Abstract
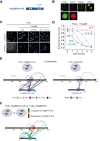
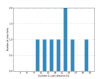
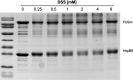
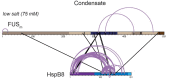
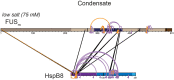
Aberrant liquid-to-solid phase transitions of biomolecular condensates have been linked to various neurodegenerative diseases. However, the underlying molecular interactions that drive aging remain enigmatic. Here, we develop quantitative time-resolved crosslinking mass spectrometry to monitor protein interactions and dynamics inside condensates formed by the protein fused in sarcoma (FUS). We identify misfolding of the RNA recognition motif of FUS as a key driver of condensate aging. We demonstrate that the small heat shock protein HspB8 partitions into FUS condensates via its intrinsically disordered domain and prevents condensate hardening via condensate-specific interactions that are mediated by its α-crystallin domain (αCD). These αCD-mediated interactions are altered in a disease-associated mutant of HspB8, which abrogates the ability of HspB8 to prevent condensate hardening. We propose that stabilizing aggregation-prone folded RNA-binding domains inside condensates by molecular chaperones may be a general mechanism to prevent aberrant phase transitions.
Keywords: FUS; RRM; aging; biochemistry; chaperones; chemical biology; human; molecular condensates; time-resolved quantitative XL-MS.
© 2021, Boczek et al.
Conflict of interest statement
EB, IP is currently an employee of Dewpoint Therapeutics, JF, MN, LJ, MJ, MR, KK, PH, LM, JW, XY, AP, DM, LH, SC No competing interests declared, SA is a shareholder, consultant and member of the scientific advisory board for Dewpoint Therapeutics, AH is cofounder, shareholder, consultant and member of the scientific advisory board for Dewpoint Therapeutics. FS is a consultant and member of the scientific advisory board for Dewpoint Therapeutics.
Figures










Comment in
-
Aggregation in the spotlight.Elife. 2021 Oct 12;10:e73586. doi: 10.7554/eLife.73586. Elife. 2021. PMID: 34636721 Free PMC article.
References
-
- Altair Developers Altair. 4.1.0GitHub. 2020 https://altair-viz.github.io/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

