The impact of childhood RSV infection on children's and parents' quality of life: a prospective multicenter study in Spain
- PMID: 34488668
- PMCID: PMC8422742
- DOI: 10.1186/s12879-021-06629-z
The impact of childhood RSV infection on children's and parents' quality of life: a prospective multicenter study in Spain
Abstract
Background: Several immunisation candidates against RSV are in late-stage clinical trials. To evaluate the benefits of a potential vaccination programme, both economic and health benefits will be needed. Health benefits are usually measured in Health-related Quality of Life (HRQoL) loss using standardised questionnaires. However, there are no RSV-specific questionnaires validated for children under 2 years, in whom most RSV episodes occur. Therefore, HRQoL estimates are taken from literature or inadequate tools. We determined HRQoL loss and direct costs due to an RSV episode in children younger than 2 years and their caregivers during a month of follow up, using a new questionnaire administered online.
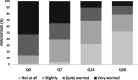
Methods: An observational prospective multicentre surveillance study was conducted in children aged younger than two years. Children were recruited from 8 primary care centres and 1 hospital in the Valencia region and Catalonia (Spain). RSV-positive cases were obtained by immunochromatographic test. HRQoL was assessed using a new ad-hoc 38 item-questionnaire developed. Parents of infected children completed 4 questionnaires at four timepoints (day 0, 7, 14 and 30) after diagnosis.
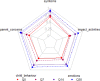
Results: 117 children were enrolled in the study and 86 (73.5%) were RSV + . Median (interquartile range; IQR) scores were 0.52 (0.42-0.68), 0.65 (0.49-0.79), 0.82 (0.68-0.97) and 0.94 (0.81-1), for days 0, 7, 14 and 30, respectively. Compared to total recovery (Q30), HRQoL loss was 37.5%, 31.5% and 8.9% on days 0, 7 and 14 since diagnosis of the disease. The total median cost per patient (including treatments) was €598.8 (IQR: 359.63-2425.85).
Conclusions: RSV had almost 40% impact on HRQoL during the first week since onset of symptoms and the median cost per episode and patient was about €600. These results represent a substantial input for health-economic evaluations of future RSV-related interventions such as vaccination.
Keywords: Children; Health‐related quality of life; Quality‐adjusted life years; Respiratory disease; Respiratory syncytial virus.
© 2021. The Author(s).
Conflict of interest statement
EDG, MLL, AOS, CMQ, IUS and JDD received travel grants to attend meetings sponsored by pharmaceutical companies. JDD has been principal investigator in clinical trials sponsored by SPMSD, MSD, GSK and Pfizer. JDD acted as Advisor for GSK and SPMSD and AOS for MSD and GSK. CGA and Study collaborators have no conflict of interest.
Figures

References
-
- Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. - DOI - PMC - PubMed
-
- RSV Vaccine Snapshot [Internet]. 2020. https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/. Accessed July 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

