APOΕ4 lowers energy expenditure in females and impairs glucose oxidation by increasing flux through aerobic glycolysis
- PMID: 34488832
- PMCID: PMC8420022
- DOI: 10.1186/s13024-021-00483-y
APOΕ4 lowers energy expenditure in females and impairs glucose oxidation by increasing flux through aerobic glycolysis
Abstract
Background: Cerebral glucose hypometabolism is consistently observed in individuals with Alzheimer's disease (AD), as well as in young cognitively normal carriers of the Ε4 allele of Apolipoprotein E (APOE), the strongest genetic predictor of late-onset AD. While this clinical feature has been described for over two decades, the mechanism underlying these changes in cerebral glucose metabolism remains a critical knowledge gap in the field.
Methods: Here, we undertook a multi-omic approach by combining single-cell RNA sequencing (scRNAseq) and stable isotope resolved metabolomics (SIRM) to define a metabolic rewiring across astrocytes, brain tissue, mice, and human subjects expressing APOE4.
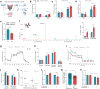
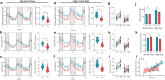
Results: Single-cell analysis of brain tissue from mice expressing human APOE revealed E4-associated decreases in genes related to oxidative phosphorylation, particularly in astrocytes. This shift was confirmed on a metabolic level with isotopic tracing of 13C-glucose in E4 mice and astrocytes, which showed decreased pyruvate entry into the TCA cycle and increased lactate synthesis. Metabolic phenotyping of E4 astrocytes showed elevated glycolytic activity, decreased oxygen consumption, blunted oxidative flexibility, and a lower rate of glucose oxidation in the presence of lactate. Together, these cellular findings suggest an E4-associated increase in aerobic glycolysis (i.e. the Warburg effect). To test whether this phenomenon translated to APOE4 humans, we analyzed the plasma metabolome of young and middle-aged human participants with and without the Ε4 allele, and used indirect calorimetry to measure whole body oxygen consumption and energy expenditure. In line with data from E4-expressing female mice, a subgroup analysis revealed that young female E4 carriers showed a striking decrease in energy expenditure compared to non-carriers. This decrease in energy expenditure was primarily driven by a lower rate of oxygen consumption, and was exaggerated following a dietary glucose challenge. Further, the stunted oxygen consumption was accompanied by markedly increased lactate in the plasma of E4 carriers, and a pathway analysis of the plasma metabolome suggested an increase in aerobic glycolysis.
Conclusions: Together, these results suggest astrocyte, brain and system-level metabolic reprogramming in the presence of APOE4, a 'Warburg like' endophenotype that is observable in young females decades prior to clinically manifest AD.
Keywords: APOE; Aerobic glycolysis; Alzheimer’s disease; Apolipoprotein E; Energy expenditure; Metabolism.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that no competing interests exist.
Figures



References
-
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease Meta analysis consortium. Jama. 1997;278(16):1349–1356. doi: 10.1001/jama.1997.03550160069041. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- P20 GM121327/GM/NIGMS NIH HHS/United States
- P30 GM127211/GM/NIGMS NIH HHS/United States
- F99 CA264165/CA/NCI NIH HHS/United States
- P30 CA177558/CA/NCI NIH HHS/United States
- RF1 NS118558/NS/NINDS NIH HHS/United States
- R01 AG060056/AG/NIA NIH HHS/United States
- T32 AG057461/AG/NIA NIH HHS/United States
- UL1 TR001998/TR/NCATS NIH HHS/United States
- R01 AG066653/AG/NIA NIH HHS/United States
- R01 AG062550/AG/NIA NIH HHS/United States
- R35 NS116824/NS/NINDS NIH HHS/United States
- T32 GM118292/GM/NIGMS NIH HHS/United States
- F30 AG063422/AG/NIA NIH HHS/United States
- R01 NS070899/NS/NINDS NIH HHS/United States
- P20 GM103527/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

