Genomic temporal heterogeneity of circulating tumour DNA in unresectable metastatic colorectal cancer under first-line treatment
- PMID: 34489309
- PMCID: PMC9185813
- DOI: 10.1136/gutjnl-2021-324852
Genomic temporal heterogeneity of circulating tumour DNA in unresectable metastatic colorectal cancer under first-line treatment
Abstract
Objective: Circulating tumour DNA (ctDNA) sequencing is increasingly used in the clinical management of patients with colorectal cancer. However, the genomic heterogeneity in ctDNA during treatments and its impact on clinical outcomes remain largely unknown.
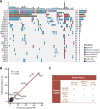
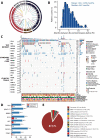
Design: We conducted a prospective cohort study (NCT04228614) of 171 patients with unresectable metastatic colorectal cancer (mCRC) who underwent first-line treatment and prospectively collected blood samples with or without tumour samples from patients at baseline and sequentially until disease progression or last follow-up.
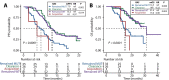
Results: The RAS/BRAF alterations in paired baseline tissue and plasma samples from 63 patients displayed a favourable concordance (81.0%, 51/63). After a period of first-line treatment (median time between baseline and last liquid biopsy, 4.67 months), 42.6% (26/61) of RAS-mutant patients showed RAS clearance and 50.0% (5/10) of BRAF-mutant patients showed BRAF clearance, while 3.6% (3/84) and 0.7% (1/135) of patients showed new RAS or BRAF mutations in ctDNA. Patients with plasma RAS/BRAF clearance showed similar progression-free survival (PFS) and overall survival (OS) with patients who remained RAS/BRAF wild-type, while much better outcomes than those who remained RAS/BRAF mutant. Patients who gained new RAS/BRAF mutations showed similar prognosis as those who maintained RAS/BRAF mutations, and shorter PFS and OS than those who remained RAS/BRAF wild-type.
Conclusion: This prospective, serial and large-scale ctDNA profiling study reveals the temporal heterogeneity of mCRC-related somatic variants, which should be given special attention in clinical practice, as evidenced by the finding that the shift in plasma RAS/BRAF mutational status can yield a drastic change in survival outcomes.
Keywords: cancer genetics; colorectal cancer genes; colorectal carcinoma.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
