Genetically encoded cell-death indicators (GEDI) to detect an early irreversible commitment to neurodegeneration
- PMID: 34489414
- PMCID: PMC8421388
- DOI: 10.1038/s41467-021-25549-9
Genetically encoded cell-death indicators (GEDI) to detect an early irreversible commitment to neurodegeneration
Abstract
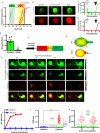
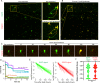
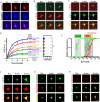
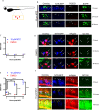
Cell death is a critical process that occurs normally in health and disease. However, its study is limited due to available technologies that only detect very late stages in the process or specific death mechanisms. Here, we report the development of a family of fluorescent biosensors called genetically encoded death indicators (GEDIs). GEDIs specifically detect an intracellular Ca2+ level that cells achieve early in the cell death process and that marks a stage at which cells are irreversibly committed to die. The time-resolved nature of a GEDI delineates a binary demarcation of cell life and death in real time, reformulating the definition of cell death. We demonstrate that GEDIs acutely and accurately report death of rodent and human neurons in vitro, and show that GEDIs enable an automated imaging platform for single cell detection of neuronal death in vivo in zebrafish larvae. With a quantitative pseudo-ratiometric signal, GEDIs facilitate high-throughput analysis of cell death in time-lapse imaging analysis, providing the necessary resolution and scale to identify early factors leading to cell death in studies of neurodegeneration.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests, but the following competing financial interests: S.F. is the inventor of Robotic Microscopy Systems, US Patent 7,139,415 and Automated Robotic Microscopy Systems, US Patent Application 14/737,325, both assigned to the J. David Gladstone Institutes. A provisional US and EPO patent for the GEDI biosensor (inventors J.W.L., K.S., and S.F.) assigned to the J. David Gladstone Institutes has been placed GL2016-815, May 2019.
Figures

References
-
- Miller J, et al. Quantitative relationships between huntingtin levels, polyglutamine length, inclusion body formation, and neuronal death provide novel insight into huntington’s disease molecular pathogenesis. J. Neurosci. 2010;30:10541–10550. doi: 10.1523/JNEUROSCI.0146-10.2010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

