Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice
- PMID: 34489419
- PMCID: PMC8421455
- DOI: 10.1038/s41467-021-25590-8
Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice
Abstract
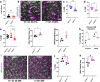
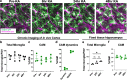
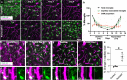
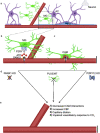
Microglia are brain-resident immune cells with a repertoire of functions in the brain. However, the extent of their interactions with the vasculature and potential regulation of vascular physiology has been insufficiently explored. Here, we document interactions between ramified CX3CR1 + myeloid cell somata and brain capillaries. We confirm that these cells are bona fide microglia by molecular, morphological and ultrastructural approaches. Then, we give a detailed spatio-temporal characterization of these capillary-associated microglia (CAMs) comparing them with parenchymal microglia (PCMs) in their morphological activities including during microglial depletion and repopulation. Molecularly, we identify P2RY12 receptors as a regulator of CAM interactions under the control of released purines from pannexin 1 (PANX1) channels. Furthermore, microglial elimination triggered capillary dilation, blood flow increase, and impaired vasodilation that were recapitulated in P2RY12-/- and PANX1-/- mice suggesting purines released through PANX1 channels play important roles in activating microglial P2RY12 receptors to regulate neurovascular structure and function.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

