C9orf72-derived arginine-rich poly-dipeptides impede phase modifiers
- PMID: 34489423
- PMCID: PMC8421406
- DOI: 10.1038/s41467-021-25560-0
C9orf72-derived arginine-rich poly-dipeptides impede phase modifiers
Abstract
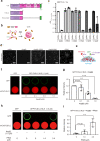
Nuclear import receptors (NIRs) not only transport RNA-binding proteins (RBPs) but also modify phase transitions of RBPs by recognizing nuclear localization signals (NLSs). Toxic arginine-rich poly-dipeptides from C9orf72 interact with NIRs and cause nucleocytoplasmic transport deficit. However, the molecular basis for the toxicity of arginine-rich poly-dipeptides toward NIRs function as phase modifiers of RBPs remains unidentified. Here we show that arginine-rich poly-dipeptides impede the ability of NIRs to modify phase transitions of RBPs. Isothermal titration calorimetry and size-exclusion chromatography revealed that proline:arginine (PR) poly-dipeptides tightly bind karyopherin-β2 (Kapβ2) at 1:1 ratio. The nuclear magnetic resonances of Kapβ2 perturbed by PR poly-dipeptides partially overlapped with those perturbed by the designed NLS peptide, suggesting that PR poly-dipeptides target the NLS binding site of Kapβ2. The findings offer mechanistic insights into how phase transitions of RBPs are disabled in C9orf72-related neurodegeneration.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




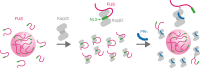
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

