Spectroscopic glimpses of the transition state of ATP hydrolysis trapped in a bacterial DnaB helicase
- PMID: 34489448
- PMCID: PMC8421360
- DOI: 10.1038/s41467-021-25599-z
Spectroscopic glimpses of the transition state of ATP hydrolysis trapped in a bacterial DnaB helicase
Abstract
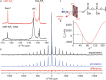
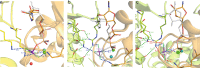
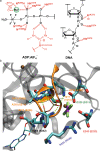
The ATP hydrolysis transition state of motor proteins is a weakly populated protein state that can be stabilized and investigated by replacing ATP with chemical mimics. We present atomic-level structural and dynamic insights on a state created by ADP aluminum fluoride binding to the bacterial DnaB helicase from Helicobacter pylori. We determined the positioning of the metal ion cofactor within the active site using electron paramagnetic resonance, and identified the protein protons coordinating to the phosphate groups of ADP and DNA using proton-detected 31P,1H solid-state nuclear magnetic resonance spectroscopy at fast magic-angle spinning > 100 kHz, as well as temperature-dependent proton chemical-shift values to prove their engagements in hydrogen bonds. 19F and 27Al MAS NMR spectra reveal a highly mobile, fast-rotating aluminum fluoride unit pointing to the capture of a late ATP hydrolysis transition state in which the phosphoryl unit is already detached from the arginine and lysine fingers.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
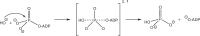



References
-
- Spies, M. DNA Helicases and DNA Motor Proteins (Springer, 2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

