MEG source imaging detects optogenetically-induced activity in cortical and subcortical networks
- PMID: 34489452
- PMCID: PMC8421372
- DOI: 10.1038/s41467-021-25481-y
MEG source imaging detects optogenetically-induced activity in cortical and subcortical networks
Abstract
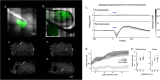
Magnetoencephalography measures neuromagnetic activity with high temporal, and theoretically, high spatial resolution. We developed an experimental platform combining MEG-compatible optogenetic techniques in nonhuman primates for use as a functional brain-mapping platform. Here we show localization of optogenetically evoked signals to known sources in the superficial arcuate sulcus of cortex and in CA3 of hippocampus at a resolution of 750 µm3. We detect activation in subcortical, thalamic, and extended temporal structures, conforming to known anatomical and functional brain networks associated with the respective sites of stimulation. This demonstrates that high-resolution localization of experimentally produced deep sources is possible within an intact brain. This approach is suitable for exploring causal relationships between discrete brain regions through precise optogenetic control and simultaneous whole brain MEG recording with high-resolution magnetic source imaging (MSI).
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Nasiotis, K., Clavagnier, S., Baillet, S. & Pack, C. C. High-resolution retinotopic maps estimated with magnetoencephalography. NeuroImage145, 107–117 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

