HSPA9/Mortalin mediates axo-protection and modulates mitochondrial dynamics in neurons
- PMID: 34489498
- PMCID: PMC8421332
- DOI: 10.1038/s41598-021-97162-1
HSPA9/Mortalin mediates axo-protection and modulates mitochondrial dynamics in neurons
Abstract
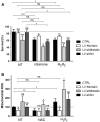
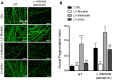
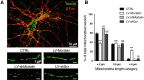
Mortalin is a mitochondrial chaperone protein involved in quality control of proteins imported into the mitochondrial matrix, which was recently described as a sensor of neuronal stress. Mortalin is down-regulated in neurons of patients with neurodegenerative diseases and levels of Mortalin expression are correlated with neuronal fate in animal models of Alzheimer's disease or cerebral ischemia. To date, however, the links between Mortalin levels, its impact on mitochondrial function and morphology and, ultimately, the initiation of neurodegeneration, are still unclear. In the present study, we used lentiviral vectors to over- or under-express Mortalin in primary neuronal cultures. We first analyzed the early events of neurodegeneration in the axonal compartment, using oriented neuronal cultures grown in microfluidic-based devices. We observed that Mortalin down-regulation induced mitochondrial fragmentation and axonal damage, whereas its over-expression conferred protection against axonal degeneration mediated by rotenone exposure. We next demonstrated that Mortalin levels modulated mitochondrial morphology by acting on DRP1 phosphorylation, thereby further illustrating the crucial implication of mitochondrial dynamics on neuronal fate in degenerative diseases.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

