Cell-autonomous megakaryopoiesis associated with polyclonal hematopoiesis in triple-negative essential thrombocythemia
- PMID: 34489506
- PMCID: PMC8421373
- DOI: 10.1038/s41598-021-97106-9
Cell-autonomous megakaryopoiesis associated with polyclonal hematopoiesis in triple-negative essential thrombocythemia
Erratum in
-
Author Correction: Cell-autonomous megakaryopoiesis associated with polyclonal hematopoiesis in triple-negative essential thrombocythemia.Sci Rep. 2022 Jan 17;12(1):1115. doi: 10.1038/s41598-022-04977-7. Sci Rep. 2022. PMID: 35039617 Free PMC article. No abstract available.
Abstract
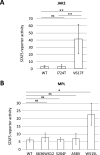
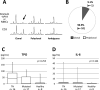
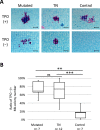
A subset of essential thrombocythemia (ET) cases are negative for disease-defining mutations on JAK2, MPL, and CALR and defined as triple negative (TN). The lack of recurrent mutations in TN-ET patients makes its pathogenesis ambiguous. Here, we screened 483 patients with suspected ET in a single institution, centrally reviewed bone marrow specimens, and identified 23 TN-ET patients. Analysis of clinical records revealed that TN-ET patients were mostly young female, without a history of thrombosis or progression to secondary myelofibrosis and leukemia. Sequencing analysis and human androgen receptor assays revealed that the majority of TN-ET patients exhibited polyclonal hematopoiesis, suggesting a possibility of reactive thrombocytosis in TN-ET. However, the serum levels of thrombopoietin (TPO) and interleukin-6 in TN-ET patients were not significantly different from those in ET patients with canonical mutations and healthy individuals. Rather, CD34-positive cells from TN-ET patients showed a capacity to form megakaryocytic colonies, even in the absence of TPO. No signs of thrombocytosis were observed before TN-ET development, denying the possibility of hereditary thrombocytosis in TN-ET. Overall, these findings indicate that TN-ET is a distinctive disease entity associated with polyclonal hematopoiesis and is paradoxically caused by hematopoietic stem cells harboring a capacity for cell-autonomous megakaryopoiesis.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

