Heart failure with mid-range or mildly reduced ejection fraction
- PMID: 34489589
- PMCID: PMC8420965
- DOI: 10.1038/s41569-021-00605-5
Heart failure with mid-range or mildly reduced ejection fraction
Abstract
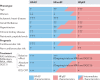
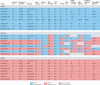
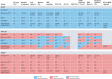
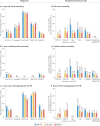
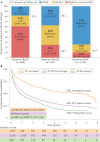
Left ventricular ejection fraction (EF) remains the major parameter for diagnosis, phenotyping, prognosis and treatment decisions in heart failure. The 2016 ESC heart failure guidelines introduced a third EF category for an EF of 40-49%, defined as heart failure with mid-range EF (HFmrEF). This category has been largely unexplored compared with heart failure with reduced EF (HFrEF; defined as EF <40% in this Review) and heart failure with preserved EF (HFpEF; defined as EF ≥50%). The prevalence of HFmrEF within the overall population of patients with HF is 10-25%. HFmrEF seems to be an intermediate clinical entity between HFrEF and HFpEF in some respects, but more similar to HFrEF in others, in particular with regard to the high prevalence of ischaemic heart disease in these patients. HFmrEF is milder than HFrEF, and the risk of cardiovascular events is lower in patients with HFmrEF or HFpEF than in those with HFrEF. By contrast, the risk of non-cardiovascular adverse events is similar or greater in patients with HFmrEF or HFpEF than in those with HFrEF. Evidence from post hoc and subgroup analyses of randomized clinical trials and a trial of an SGLT1-SGLT2 inhibitor suggests that drugs that are effective in patients with HFrEF might also be effective in patients with HFmrEF. Although the EF is a continuous measure with considerable variability, in this comprehensive Review we suggest that HFmrEF is a useful categorization of patients with HF and shares the most important clinical features with HFrEF, which supports the renaming of HFmrEF to HF with mildly reduced EF.
© 2021. Springer Nature Limited.
Conflict of interest statement
G. Savarese reports grants and personal fees from AstraZeneca and Vifor; grants and non-financial support from Boehringer Ingelheim; personal fees from Cytokinetics, GENESIS, Medtronic, Radcliffe, Roche, Servier and Società Prodotti Antibiotici; and grants from Boston Scientific, Novartis and Pharmacosmos, unrelated to this Review. D.S. reports personal fees from GlaxoSmithKline, MSD and Novartis. L.H.L. reports personal fees from Abbott, Bayer, Lexicon, Medscape, Merck, Myokardia, Pharmacosmos, Radcliffe Cardiology and Sanofi; grants and personal fees from AstraZeneca, Boehringer Ingelheim, Novartis and Vifor–Fresenius; and grants from Boston Scientific, unrelated to this Review. G. Sinagra declares no competing interests.
Figures
References
-
- Yancy CW, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

