Corticothalamic Pathways in Auditory Processing: Recent Advances and Insights From Other Sensory Systems
- PMID: 34489648
- PMCID: PMC8418311
- DOI: 10.3389/fncir.2021.721186
Corticothalamic Pathways in Auditory Processing: Recent Advances and Insights From Other Sensory Systems
Abstract
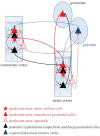
The corticothalamic (CT) pathways emanate from either Layer 5 (L5) or 6 (L6) of the neocortex and largely outnumber the ascending, thalamocortical pathways. The CT pathways provide the anatomical foundations for an intricate, bidirectional communication between thalamus and cortex. They act as dynamic circuits of information transfer with the ability to modulate or even drive the response properties of target neurons at each synaptic node of the circuit. L6 CT feedback pathways enable the cortex to shape the nature of its driving inputs, by directly modulating the sensory message arriving at the thalamus. L5 CT pathways can drive the postsynaptic neurons and initiate a transthalamic corticocortical circuit by which cortical areas communicate with each other. For this reason, L5 CT pathways place the thalamus at the heart of information transfer through the cortical hierarchy. Recent evidence goes even further to suggest that the thalamus via CT pathways regulates functional connectivity within and across cortical regions, and might be engaged in cognition, behavior, and perceptual inference. As descending pathways that enable reciprocal and context-dependent communication between thalamus and cortex, we venture that CT projections are particularly interesting in the context of hierarchical perceptual inference formulations such as those contemplated in predictive processing schemes, which so far heavily rely on cortical implementations. We discuss recent proposals suggesting that the thalamus, and particularly higher order thalamus via transthalamic pathways, could coordinate and contextualize hierarchical inference in cortical hierarchies. We will explore these ideas with a focus on the auditory system.
Keywords: corticothalamic circuits; feedback loops; hierarchical inference; reticular thalamic nucleus; transthalamic pathways.
Copyright © 2021 Antunes and Malmierca.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

