Chimeric Antigen Receptor-T Cells: A Pharmaceutical Scope
- PMID: 34489708
- PMCID: PMC8417740
- DOI: 10.3389/fphar.2021.720692
Chimeric Antigen Receptor-T Cells: A Pharmaceutical Scope
Abstract
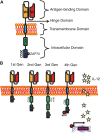
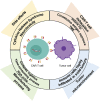
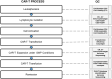
Cancer is among the leading causes of death worldwide. Therefore, improving cancer therapeutic strategies using novel alternatives is a top priority on the contemporary scientific agenda. An example of such strategies is immunotherapy, which is based on teaching the immune system to recognize, attack, and kill malignant cancer cells. Several types of immunotherapies are currently used to treat cancer, including adoptive cell therapy (ACT). Chimeric Antigen Receptors therapy (CAR therapy) is a kind of ATC where autologous T cells are genetically engineered to express CARs (CAR-T cells) to specifically kill the tumor cells. CAR-T cell therapy is an opportunity to treat patients that have not responded to other first-line cancer treatments. Nowadays, this type of therapy still has many challenges to overcome to be considered as a first-line clinical treatment. This emerging technology is still classified as an advanced therapy from the pharmaceutical point of view, hence, for it to be applied it must firstly meet certain requirements demanded by the authority. For this reason, the aim of this review is to present a global vision of different immunotherapies and focus on CAR-T cell technology analyzing its elements, its history, and its challenges. Furthermore, analyzing the opportunity areas for CAR-T technology to become an affordable treatment modality taking the basic, clinical, and practical aspects into consideration.
Keywords: CAR-T cells therapy; advanced cell therapy; cell and gene therapy; cell manufacturing process; immunotherapy.
Copyright © 2021 Hernández-López, Téllez-González, Mondragón-Terán and Meneses-Acosta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Aftab B. T., Sasu B., Krishnamurthy J., Gschweng E., Alcazer V., Depil S. (2020). Toward "Off-The-Shelf" Allogeneic CAR T Cells. Adv. Cel Gene Ther 3, 1–11. 10.1002/acg2.86 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

