Update in Autoimmune Movement Disorders: Newly Described Antigen Targets in Autoimmune and Paraneoplastic Cerebellar Ataxia
- PMID: 34489848
- PMCID: PMC8416494
- DOI: 10.3389/fneur.2021.683048
Update in Autoimmune Movement Disorders: Newly Described Antigen Targets in Autoimmune and Paraneoplastic Cerebellar Ataxia
Abstract
Movement disorders are a common feature of many antibody-associated neurological disorders. In fact, cerebellar ataxia is one of the most common manifestations of autoimmune neurological diseases. Some of the first autoantibodies identified against antigen targets include anti-neuronal nuclear antibody type 1 (ANNA-1 or anti-Hu) and Purkinje cell cytoplasmic antibody (PCA-1) also known as anti-Yo have been identified in paraneoplastic cerebellar degeneration. Historically these antibodies have been associated with an underlying malignancy; however, recently discovered antibodies can occur in the absence of cancer as well, resulting in the clinical syndrome of autoimmune cerebellar ataxia. The pace of discovery of new antibodies associated with autoimmune or paraneoplastic cerebellar ataxia has increased rapidly over the last few years, and pathogenesis and potential treatment options remains to be explored. Here we will review the literature on recently discovered antibodies associated with autoimmune and paraneoplastic cerebellar ataxia including adaptor protein-3B2 (AP3B2); inositol 1,4,5-trisphophate receptor type 1 (ITPR1); tripartite motif-containing (TRIM) proteins 9, 67, and 46; neurochondrin; neuronal intermediate filament light chain (NIF); septin 5; metabotropic glutamate receptor 2 (mGluR2); seizure-related 6 homolog like 2 (SEZ6L2) and homer-3 antibodies. We will review their clinical characteristics, imaging and CSF findings and treatment response. In addition, we will discuss two clinical case examples of autoimmune cerebellar ataxia.
Keywords: AP3B2 antibody; ITPR1 antibody; TRIM46 antibody; TRIM67 antibody; TRIM9 antibody; autoimmune cerebellar ataxia; mGluR2 antibody; paraneoplastic cerebellar ataxia.
Copyright © 2021 Garza and Piquet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
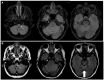
Figures


References
-
- Lopez-Chiriboga AS, McKeon A. Autoimmune and paraneoplastic movement disorders. In: Piquet A, Alvarez E, editors. Neuroimmunology: Multiple Sclerosis, Autoimmune Neurology and Related Disease. 1. Switzerland: Springer Nature. (2021). p. 207–20. 10.1007/978-3-030-61883-4_14 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

