Deciphering the Antibacterial Role of Peptide From Bacillus subtilis subsp. spizizenii Ba49 Against Staphylococcus aureus
- PMID: 34489898
- PMCID: PMC8417246
- DOI: 10.3389/fmicb.2021.708712
Deciphering the Antibacterial Role of Peptide From Bacillus subtilis subsp. spizizenii Ba49 Against Staphylococcus aureus
Abstract
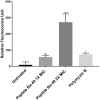
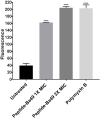
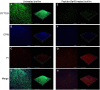
An increase in antibiotic resistance has led to escalating the need for the development of alternate therapy. Antimicrobial peptides (AMPs) are at the forefront of replacing conventional antibiotics, showing slower development of drug resistance, antibiofilm activity, and the ability to modulate the host immune response. The ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogens that jeopardize most conventional antibiotics are known to be involved in severe respiratory tract, bloodstream, urinary tract, soft tissue, and skin infections. Among them, S. aureus is an insidious microbe and developed resistance against conventional antibiotics. In the present study, an AMP (named as peptide-Ba49) isolated from Bacillus subtilis subsp. spizizenii strain from Allium cepa (the common onion) exhibited strong antibacterial efficacy against S. aureus ATCC 25923. The mode of action of this peptide-Ba49 on S. aureus was deciphered through various sensitive probes, i.e., DiSC3 (5) and H2DCFDA, suggesting the peptide-Ba49 to be acting upon through change in membrane potential and by triggering the production of reactive oxygen species (ROS). This induced disruption of the cell membrane was further supported by morphological studies using scanning electron microscopy (SEM). Investigations on a possible post-antibiotic effect (PAE) of peptide-Ba49 showed prolonged PAE against S. aureus. Furthermore, the peptide-Ba49 prevented the formation of S. aureus biofilm at low concentration and showed its potential to degrade the mature biofilm of S. aureus. The peptide-Ba49 also exhibited intracellular killing potential against S. aureus ATCC 25923 in the macrophage cells, and moreover, peptide-Ba49 was found to bolster the fibroblast cell migration in the scratch assay at low concentration, exhibiting a wound healing efficacy of this peptide. These studies demonstrated that peptide-Ba49 isolated from the strain B. subtilis subsp. spizizenii could be a therapeutic candidate to combat the pathogenic S. aureus infections.
Keywords: PAE; ROS; Staphylococcus aureus; antimicrobial peptides; biofilm; intracellular activity; scratch assay.
Copyright © 2021 Taggar, Singh, Bhalla, Bhattacharyya and Sahoo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
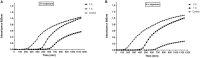






Similar articles
-
Bacteriocin isolated from the natural inhabitant of Allium cepa against Staphylococcus aureus.World J Microbiol Biotechnol. 2021 Jan 11;37(2):20. doi: 10.1007/s11274-020-02989-x. World J Microbiol Biotechnol. 2021. PMID: 33427970
-
Human Salivary Protein Histatin 5 Has Potent Bactericidal Activity against ESKAPE Pathogens.Front Cell Infect Microbiol. 2017 Feb 15;7:41. doi: 10.3389/fcimb.2017.00041. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28261570 Free PMC article.
-
Norfloxacin salts of carboxylic acids curtail planktonic and biofilm mode of growth in ESKAPE pathogens.J Appl Microbiol. 2018 Feb;124(2):408-422. doi: 10.1111/jam.13651. J Appl Microbiol. 2018. PMID: 29178633
-
Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds.Front Pharmacol. 2018 Mar 28;9:281. doi: 10.3389/fphar.2018.00281. eCollection 2018. Front Pharmacol. 2018. PMID: 29643807 Free PMC article. Review.
-
Emerging peptide antibiotics with therapeutic potential.Med Drug Discov. 2021 Mar;9:100078. doi: 10.1016/j.medidd.2020.100078. Epub 2020 Dec 30. Med Drug Discov. 2021. PMID: 33398258 Free PMC article. Review.
Cited by
-
Diversity and Mechanisms of Action of Plant, Animal, and Human Antimicrobial Peptides.Antibiotics (Basel). 2024 Feb 21;13(3):202. doi: 10.3390/antibiotics13030202. Antibiotics (Basel). 2024. PMID: 38534637 Free PMC article. Review.
-
Microbial synthesis of enantiopure (S)-2-methylbutanoic acid via L-isoleucine catabolism in Bacillus spizizenii.World J Microbiol Biotechnol. 2025 Mar 28;41(4):117. doi: 10.1007/s11274-025-04324-8. World J Microbiol Biotechnol. 2025. PMID: 40148725
-
Antimicrobial activities of lavandulylated flavonoids in Sophora flavences against methicillin-resistant Staphylococcus aureus via membrane disruption.J Adv Res. 2024 Mar;57:197-212. doi: 10.1016/j.jare.2023.04.017. Epub 2023 May 1. J Adv Res. 2024. PMID: 37137428 Free PMC article.
-
Probiotics and Their Bioproducts: A Promising Approach for Targeting Methicillin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus.Microorganisms. 2023 Sep 25;11(10):2393. doi: 10.3390/microorganisms11102393. Microorganisms. 2023. PMID: 37894051 Free PMC article. Review.
-
A truncated peptide Spgillcin177-189 derived from mud crab Scylla paramamosain exerting multiple antibacterial activities.Front Cell Infect Microbiol. 2022 Aug 2;12:928220. doi: 10.3389/fcimb.2022.928220. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36061863 Free PMC article.
References
-
- Barrett L., Atkins B. (2014). The clinical presentation of prosthetic joint infection. J. Antimicrob. Chemother. 69(Suppl. 1), i25–i27. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

