Combining Immunocytokine and Ex Vivo Activated NK Cells as a Platform for Enhancing Graft-Versus-Tumor Effects Against GD2+ Murine Neuroblastoma
- PMID: 34489927
- PMCID: PMC8417312
- DOI: 10.3389/fimmu.2021.668307
Combining Immunocytokine and Ex Vivo Activated NK Cells as a Platform for Enhancing Graft-Versus-Tumor Effects Against GD2+ Murine Neuroblastoma
Abstract
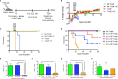
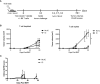
Management for high-risk neuroblastoma (NBL) has included autologous hematopoietic stem cell transplant (HSCT) and anti-GD2 immunotherapy, but survival remains around 50%. The aim of this study was to determine if allogeneic HSCT could serve as a platform for inducing a graft-versus-tumor (GVT) effect against NBL with combination immunocytokine and NK cells in a murine model. Lethally irradiated C57BL/6 (B6) x A/J recipients were transplanted with B6 bone marrow on Day +0. On day +10, allogeneic HSCT recipients were challenged with NXS2, a GD2+ NBL. On days +14-16, mice were treated with the anti-GD2 immunocytokine hu14.18-IL2. In select groups, hu14.18-IL2 was combined with infusions of B6 NK cells activated with IL-15/IL-15Rα and CD137L ex vivo. Allogeneic HSCT alone was insufficient to control NXS2 tumor growth, but the addition of hu14.18-IL2 controlled tumor growth and improved survival. Adoptive transfer of ex vivo CD137L/IL-15/IL-15Rα activated NK cells with or without hu14.18-IL2 exacerbated lethality. CD137L/IL-15/IL-15Rα activated NK cells showed enhanced cytotoxicity and produced high levels of TNF-α in vitro, but induced cytokine release syndrome (CRS) in vivo. Infusing Perforin-/- CD137L/IL-15/IL-15Rα activated NK cells had no impact on GVT, whereas TNF-α-/- CD137L/IL-15/IL-15Rα activated NK cells improved GVT by decreasing peripheral effector cell subsets while preserving tumor-infiltrating lymphocytes. Depletion of Ly49H+ NK cells also improved GVT. Using allogeneic HSCT for NBL is a viable platform for immunocytokines and ex vivo activated NK cell infusions, but must be balanced with induction of CRS. Regulation of TNFα or activating NK subsets may be needed to improve GVT effects.
Keywords: NK cells; cytokine release syndrome; graft-versus-tumor effect; immunocytokine; neuroblastoma.
Copyright © 2021 Bates, Rakhmilevich, Cho, Bouchlaka, Rao, Hales, Orentas, Fry, Gilles, Sondel and Capitini.
Conflict of interest statement
SG is an employee of Provenance Biopharmaceuticals, and has a patent related to hu14.18-IL2. CC reports honorarium from Nektar Therapeutics and Novartis. These companies had no input in the study design, analysis, manuscript preparation or decision to submit for publication. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Philip T, Ladenstein R, Lasset C, Hartmann O, Zucker JM, Pinkerton R, et al. 1070 Myeloablative Megatherapy Procedures Followed by Stem Cell Rescue for Neuroblastoma: 17 Years of European Experience and Conclusions. European Group for Blood and Marrow Transplant Registry Solid Tumour Working Party. Eur J Cancer (1997) 33(12):2130–5. 10.1016/s0959-8049(97)00324-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

