The Role of Non-Immune Cell-Derived Extracellular Vesicles in Allergy
- PMID: 34489951
- PMCID: PMC8417238
- DOI: 10.3389/fimmu.2021.702381
The Role of Non-Immune Cell-Derived Extracellular Vesicles in Allergy
Abstract
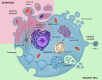
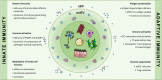
Extracellular vesicles (EVs), and especially exosomes, have been shown to mediate information exchange between distant cells; this process directly affects the biological characteristics and functionality of the recipient cell. As such, EVs significantly contribute to the shaping of immune responses in both physiology and disease states. While vesicles secreted by immune cells are often implicated in the allergic process, growing evidence indicates that EVs from non-immune cells, produced in the stroma or epithelia of the organs directly affected by inflammation may also play a significant role. In this review, we provide an overview of the mechanisms of allergy to which those EVs contribute, with a particular focus on small EVs (sEVs). Finally, we also give a clinical perspective regarding the utilization of the EV-mediated communication route for the benefit of allergic patients.
Keywords: allergic rhinitis; allergy; asthma; atopic dermatitis; cellular communication; exosomes; extracellular vesicles; immune responses.
Copyright © 2021 Hovhannisyan, Czechowska and Gutowska-Owsiak.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

