The Pathophysiology and Treatment of Graft- Versus-Host Disease: Lessons Learnt From Animal Models
- PMID: 34489966
- PMCID: PMC8417310
- DOI: 10.3389/fimmu.2021.715424
The Pathophysiology and Treatment of Graft- Versus-Host Disease: Lessons Learnt From Animal Models
Abstract
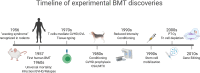
Allogeneic hematopoietic cell transplantation (HCT) is a curative treatment for hematologic malignancies, bone marrow failure syndromes, and inherited immunodeficiencies and metabolic diseases. Graft-versus-host disease (GVHD) is the major life-threatening complication after allogeneic HCT. New insights into the pathophysiology of GVHD garnered from our understanding of the immunological pathways within animal models have been pivotal in driving new therapeutic paradigms in the clinic. Successful clinical translations include histocompatibility matching, GVHD prophylaxis using cyclosporine and methotrexate, posttransplant cyclophosphamide, and the use of broad kinase inhibitors that inhibit cytokine signaling (e.g. ruxolitinib). New approaches focus on naïve T cell depletion, targeted cytokine modulation and the inhibition of co-stimulation. This review highlights the use of animal transplantation models to guide new therapeutic principles.
Keywords: animal models; graft-versus-host disease; history; pathophysiology; treatment.
Copyright © 2021 Teshima and Hill.
Conflict of interest statement
TT: Grants from Kyowa Kirin, Chugai, Sanofi, Astellas, TEIJIN PHARMA, Fuji Pharma, NIPPON SHINYAKU, Personal Fees from Novartis, Merck, Kyowa Kirin, Takeda, Pfizer, Bristol-Myers Squibb, Non-Financial Support from Janssen, Novartis. GRH has consulted for Generon Corporation, NapaJen Pharma, Neoleukin Therapeutics, iTeos Therapeutics and has received research funding from Roche Pharmaceuticals, Compass Therapeutics, Syndax Pharmaceuticals, Applied Molecular Transport and iTeos Therapeutics.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

