Decreased USP2a Expression Inhibits Trophoblast Invasion and Associates With Recurrent Miscarriage
- PMID: 34489969
- PMCID: PMC8416978
- DOI: 10.3389/fimmu.2021.717370
Decreased USP2a Expression Inhibits Trophoblast Invasion and Associates With Recurrent Miscarriage
Abstract
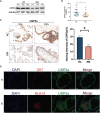
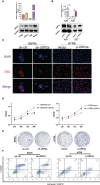
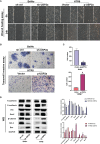
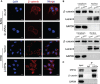
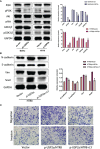
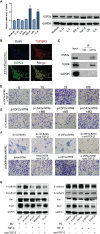
An appropriate development of the placenta consisting of trophoblast cell migration, invasion, proliferation, and apoptosis, is essential to establishing and maintaining a successful pregnancy. Ubiquitin-specific protease 2a (USP2a) regulates the processes of metastasis in multiple tumor cells. Yet, no known research has focused on exploring the effect of USP2a on trophoblasts and its possible mechanism in the pathogenies of recurrent miscarriage (RM). In this study, we first detected the decreased mRNA levels and the protein levels of USP2a in placental villous tissue samples from the RM patients. In vitro assays verified that overexpression of USP2a promoted human trophoblast proliferation, migration, invasion, whereas knockdown of USP2a inhibited these processes. Mechanistically, USP2a activated PI3K/Akt/GSK3β signaling pathway to promote nuclear translocation of β-catenin and further activated epithelial-mesenchymal transition (EMT) in the trophoblasts. Moreover, transforming growth factor-beta (TGF-β) up-regulated USP2a expression in trophoblasts. Interestingly, M2 macrophage secreted TGF-β induced trophoblast migration and invasion, and an anti-TGF-β antibody alleviated this effect. Collectively, this study indicated that USP2a regulated trophoblast invasion and that abnormal USP2a expression might lead to aberrant trophoblast invasion, thus contributing to RM.
Keywords: TGF-β; USP2a; decidual macrophage; recurrent miscarriage; trophoblast invasion.
Copyright © 2021 Wang, Ding, Zhang, Chen, Yan, Zhang and Yin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

