Identification of PAFAH1B3 as Candidate Prognosis Marker and Potential Therapeutic Target for Hepatocellular Carcinoma
- PMID: 34490100
- PMCID: PMC8418329
- DOI: 10.3389/fonc.2021.700700
Identification of PAFAH1B3 as Candidate Prognosis Marker and Potential Therapeutic Target for Hepatocellular Carcinoma
Abstract
Background: Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related deaths worldwide. PAFAH1B3 plays an important role on occurrence and development in a variety tumor. However, the function of PAFAH1B3 in HCC remains unclear.
Methods: The TIMER, ONCOMINE, Human Protein Atlas (HPA), GEPIA, The Cancer Genome Atlas (TCGA), HCCDB, UALCAN and LinkedOmics database were used to analyze the prognostic value, co-expression genes and regulator networks of PAFAH1B3 in HCC. siRNA transfections and inhibitor of PAFAH1B3 P11 were used to verify the anti-tumor effect on HCC cell lines. Gene expression was detected by qRT-PCR. The functions of PAFAH1B3 downregulation in HCC cell lines were investigated using cell cycle analysis, apoptosis detection, CCK8 assay and transwell assay. Western blot was used to evaluate the role of PAFAH1B3 on metabolic pathways in HCC cells.
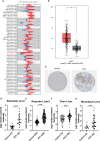
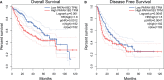
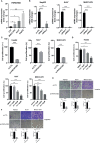
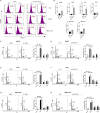
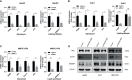
Results: Based on the data from databases, the expression of PAFAH1B3 was remarkably increased in HCC patients. High expression of PAFAH1B3 was associated with poorer overall survival (OS) and disease-free survival (DFS). And PAFAH1B3 was notably linked to age, sex, grade, stage, race, and TP53 mutational status. Then, the functional network analysis showed PAFAH1B3 may be involved in HCC through cell cycle, cell metabolism, spliceosome, and RNA transport. Furthermore, the mRNA expression of PAFAH1B3 was also increased in HCC cell lines. Flow cytometry analysis showed that PAFAH1B3 manipulated apoptosis and cell cycle regulation. CCK8 assay showed that PAFAH1B3 silencing or pharmacologic inhibitor of PAFAH1B3 inhibited the proliferation of HepG2, Huh7 and MHCC-97H cells. Transwell assay results showed that PAFAH1B3 silencing also significantly impaired the invasion and migratory ability of HCC cells. In addition, PAFAH1B3 silencing significantly downregulated the expression of glycolysis and lipid synthesis signaling pathways.
Conclusion: Our findings suggested that PAFAH1B3 plays a critical role in progression of HCC. PAFAH1B3 as a prognosis marker and potential target for HCC has prospective clinical significance.
Keywords: PAFAH1B3; biomarker; cancer databases; hepatocellular carcinoma; prognosis.
Copyright © 2021 Xu, Lu, Liu, Chen, Huang, Huang, Liu, Zhu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

