GZ17-6.02 and Pemetrexed Interact to Kill Osimertinib-Resistant NSCLC Cells That Express Mutant ERBB1 Proteins
- PMID: 34490108
- PMCID: PMC8417372
- DOI: 10.3389/fonc.2021.711043
GZ17-6.02 and Pemetrexed Interact to Kill Osimertinib-Resistant NSCLC Cells That Express Mutant ERBB1 Proteins
Abstract
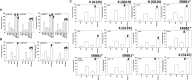
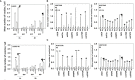
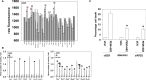
We determined the molecular mechanisms by which the novel therapeutic GZ17-6.02 killed non-small cell lung cancer (NSCLC) cells. Erlotinib, afatinib, and osimertinib interacted with GZ17-6.02 to kill NSCLC cells expressing mutant EGFR proteins. GZ17-6.02 did not interact with any EGFR inhibitor to kill osimertinib-resistant cells. GZ17-6.02 interacted with the thymidylate synthase inhibitor pemetrexed to kill NSCLC cells expressing mutant ERBB1 proteins or mutant RAS proteins or cells that were resistant to EGFR inhibitors. The drugs interacted to activate ATM, the AMPK, and ULK1 and inactivate mTORC1, mTORC2, ERK1/2, AKT, eIF2α; and c-SRC. Knockdown of ATM or AMPKα1 prevented ULK1 activation. The drugs interacted to cause autophagosome formation followed by flux, which was significantly reduced by knockdown of ATM, AMPKα1, and eIF2α, or by expression of an activated mTOR protein. Knockdown of Beclin1, ATG5, or [BAX + BAK] partially though significantly reduced drug combination lethality as did expression of activated mTOR/AKT/MEK1 or over-expression of BCL-XL. Expression of dominant negative caspase 9 weakly reduced killing. The drug combination reduced the expression of HDAC2 and HDAC3, which correlated with lower PD-L1, IDO1, and ODC levels and increased MHCA expression. Collectively, our data support consideration of combining GZ17-6.02 and pemetrexed in osimertinib-resistant NSCLC.
Keywords: EGFR; ER stress; GZ17-6.02; NSCLC; autophagy; osimertinib; pemetrexed; resistance.
Copyright © 2021 Booth, West, Moore, Von Hoff and Dent.
Conflict of interest statement
PD has received funding from Genzada Pharmaceuticals Inc. PM and CW are paid officers of the company. PD and DH are key company scientific advisors/consultants. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Figures
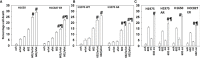
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

