Comprehensive Pan-Cancer Analysis and the Regulatory Mechanism of ASF1B, a Gene Associated With Thyroid Cancer Prognosis in the Tumor Micro-Environment
- PMID: 34490109
- PMCID: PMC8417739
- DOI: 10.3389/fonc.2021.711756
Comprehensive Pan-Cancer Analysis and the Regulatory Mechanism of ASF1B, a Gene Associated With Thyroid Cancer Prognosis in the Tumor Micro-Environment
Abstract
Background: The incidence of thyroid cancer, whose local recurrence and metastasis lead to death, has always been high and the pathogenesis of papillary thyroid carcinoma (PTC) has not been clearly elucidated. Therefore, the research for more accurate prognosis-related predictive biomarkers is imminent, and a key gene can often be a prognostic marker for multiple tumors.
Methods: Gene expression profiles of various cancers in the TCGA and GTEx databases were downloaded, and genes significantly associated with the prognosis of THCA were identified by combining differential analysis with survival analysis. Then, a series of bioinformatics tools and methods were used to analyze the expression of the gene in each cancer and the correlation of each expression with prognosis, tumor immune microenvironment, immune neoantigens, immune checkpoints, DNA repair genes, and methyltransferases respectively. The possible biological mechanisms were also investigated by GSEA enrichment analysis.
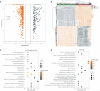
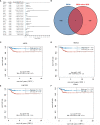
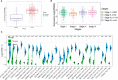
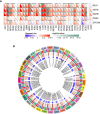
Results: 656 differentially expressed genes were identified from two datasets and 960 DEGs that were associated with disease-free survival in THCA patients were screened via survival analysis. The former and the latter were crossed to obtain 7 key genes, and the gene with the highest risk factor, ASF1B, was selected for this study. Differential analysis of multiple databases showed that ASF1B was commonly and highly expressed in pan-cancer. Survival analysis showed that high ASF1B expression was significantly associated with poor patient prognosis in multiple cancers. In addition, ASF1B expression levels were found to be associated with tumor immune infiltration in THCA, KIRC, LGG, and LIHC, and with tumor microenvironment in BRCA, LUSC, STAD, UCEC, and KIRC. Further analysis of the relationship between ASF1B expression and immune checker gene expression suggested that ASF1B may regulate tumor immune patterns in most tumors by regulating the expression levels of specific immune checker genes. Finally, GSEA enrichment analysis showed that ASF1B high expression was mainly enriched in cell cycle, MTORC1 signaling system, E2F targets, and G2M checkpoints pathways.
Conclusions: ASF1B may be an independent prognostic marker for predicting the prognosis of THCA patients. The pan-cancer analysis suggested that ASF1B may play an important role in the tumor micro-environment and tumor immunity and it has the potential of serving as a predictive biomarker for multiple cancers.
Keywords: anti-silencing function protein 1 homolog B (ASF1B); pan-cancer; prognosis; thyroid cancer; tumor immune micro-environment.
Copyright © 2021 Ma, Han and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More “Personalized” Approach to Cancer Staging. CA: Cancer J Clin (2017) 67(2):93–9. 10.3322/caac.21388 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

