The Role of Gut Microbiota in Lung Cancer: From Carcinogenesis to Immunotherapy
- PMID: 34490119
- PMCID: PMC8417127
- DOI: 10.3389/fonc.2021.720842
The Role of Gut Microbiota in Lung Cancer: From Carcinogenesis to Immunotherapy
Abstract
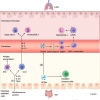
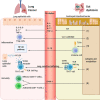
The influence of microbiota on host health and disease has attracted adequate attention, and gut microbiota components and microbiota-derived metabolites affect host immune homeostasis locally and systematically. Some studies have found that gut dysbiosis, disturbance of the structure and function of the gut microbiome, disrupts pulmonary immune homeostasis, thus leading to increased disease susceptibility; the gut-lung axis is the primary cross-talk for this communication. Gut dysbiosis is involved in carcinogenesis and the progression of lung cancer through genotoxicity, systemic inflammation, and defective immunosurveillance. In addition, the gut microbiome harbors the potential to be a novel biomarker for predicting sensitivity and adverse reactions to immunotherapy in patients with lung cancer. Probiotics and fecal microbiota transplantation (FMT) can enhance the efficacy and depress the toxicity of immune checkpoint inhibitors by regulating the gut microbiota. Although current studies have found that gut microbiota closely participates in the development and immunotherapy of lung cancer, the mechanisms require further investigation. Therefore, this review aims to discuss the underlying mechanisms of gut microbiota influencing carcinogenesis and immunotherapy in lung cancer and to provide new strategies for governing gut microbiota to enhance the prevention and treatment of lung cancer.
Keywords: biomarker; gut microbiota; gut-lung axis; immunotherapy; lung cancer.
Copyright © 2021 Liu, Cheng, Zang, Zhang, Li, Liu, Gao, Zhou, Sun, Han, Lin and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
[The Role of Gut Microbiota in Lung Carcinogenesis and Cancer Immunotherapy].Gan To Kagaku Ryoho. 2024 Jun;51(6):597-602. Gan To Kagaku Ryoho. 2024. PMID: 39009513 Japanese.
-
Modulatory effects of gut microbiome in cancer immunotherapy: A novel paradigm for blockade of immune checkpoint inhibitors.Cancer Med. 2021 Feb;10(3):1141-1154. doi: 10.1002/cam4.3694. Epub 2020 Dec 25. Cancer Med. 2021. PMID: 33369247 Free PMC article. Review.
-
Gut microbiome and response to checkpoint inhibitors in non-small cell lung cancer-A review.Crit Rev Oncol Hematol. 2020 Jan;145:102841. doi: 10.1016/j.critrevonc.2019.102841. Epub 2019 Dec 23. Crit Rev Oncol Hematol. 2020. PMID: 31884204 Review.
-
Exploring the complex and multifaceted interplay of the gut microbiome and cancer prevention and therapy.Acta Microbiol Immunol Hung. 2023 Jun 12;70(2):85-99. doi: 10.1556/030.2023.02054. Print 2023 Jun 16. Acta Microbiol Immunol Hung. 2023. PMID: 37307215 Review.
-
Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies.J Hematol Oncol. 2022 Apr 29;15(1):47. doi: 10.1186/s13045-022-01273-9. J Hematol Oncol. 2022. PMID: 35488243 Free PMC article. Review.
Cited by
-
Food System Transformation and Gut Microbiota Transition: Evidence on Advancing Obesity, Cardiovascular Diseases, and Cancers-A Narrative Review.Foods. 2023 Jun 6;12(12):2286. doi: 10.3390/foods12122286. Foods. 2023. PMID: 37372497 Free PMC article. Review.
-
A Combination of Two Probiotics, Lactobacillus sporogenes and Clostridium butyricum, Inhibits Colon Cancer Development: An In Vitro Study.Microorganisms. 2022 Aug 23;10(9):1692. doi: 10.3390/microorganisms10091692. Microorganisms. 2022. PMID: 36144294 Free PMC article.
-
The Complex Role of the Microbiome in Non-Small Cell Lung Cancer Development and Progression.Cells. 2023 Dec 8;12(24):2801. doi: 10.3390/cells12242801. Cells. 2023. PMID: 38132121 Free PMC article. Review.
-
Obesity and lung cancer - is programmed death ligand-1 (PD-1L) expression a connection?Arch Med Sci. 2024 Jan 12;20(1):313-316. doi: 10.5114/aoms/175470. eCollection 2024. Arch Med Sci. 2024. PMID: 38414472 Free PMC article.
-
A new perspective on gut-lung axis affected through resident microbiome and their implications on immune response in respiratory diseases.Arch Microbiol. 2024 Feb 18;206(3):107. doi: 10.1007/s00203-024-03843-6. Arch Microbiol. 2024. PMID: 38368569 Review.
References
Publication types
LinkOut - more resources
Full Text Sources

