Molecular Characterization of Microbiota in Cerebrospinal Fluid From Patients With CSF Shunt Infections Using Whole Genome Amplification Followed by Shotgun Sequencing
- PMID: 34490140
- PMCID: PMC8417900
- DOI: 10.3389/fcimb.2021.699506
Molecular Characterization of Microbiota in Cerebrospinal Fluid From Patients With CSF Shunt Infections Using Whole Genome Amplification Followed by Shotgun Sequencing
Abstract
Understanding the etiology of cerebrospinal fluid (CSF) shunt infections and reinfections requires detailed characterization of associated microorganisms. Traditionally, identification of bacteria present in the CSF has relied on culture methods, but recent studies have used high throughput sequencing of 16S rRNA genes. Here we evaluated the method of shotgun DNA sequencing for its potential to provide additional genomic information. CSF samples were collected from 3 patients near the beginning and end of each of 2 infection episodes. Extracted total DNA was sequenced by: (1) whole genome amplification followed by shotgun sequencing (WGA) and (2) high-throughput sequencing of the 16S rRNA V4 region (16S). Taxonomic assignments of sequences from WGA and 16S were compared with one another and with conventional microbiological cultures. While classification of bacteria was consistent among the 3 approaches, WGA provided additional insights into sample microbiological composition, such as showing relative abundances of microbial versus human DNA, identifying samples of questionable quality, and detecting significant viral load in some samples. One sample yielded sufficient non-human reads to allow assembly of a high-quality Staphylococcus epidermidis genome, denoted CLIMB1, which we characterized in terms of its MLST profile, gene complement (including putative antimicrobial resistance genes), and similarity to other annotated S. epidermidis genomes. Our results demonstrate that WGA directly applied to CSF is a valuable tool for the identification and genomic characterization of dominant microorganisms in CSF shunt infections, which can facilitate molecular approaches for the development of better diagnostic and treatment methods.
Keywords: CSF shunt infection; Staphylococcus epidermidis CLIMB1; cerebrospinal fluid; high throughput DNA sequencing; microbiota.
Copyright © 2021 Hodor, Pope, Whitlock, Hoffman, Limbrick, McDonald, Hauptman, Ojemann and Simon.
Conflict of interest statement
Author KW was employed by company New Harmony Statistical Consulting LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
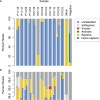
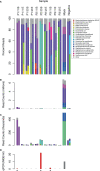
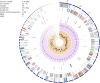

References
-
- Baron E. J., Miller J. M., Weinstein M. P., Richter S. S., Gilligan P. H., Thomson R. B., Jr., et al. (2013). A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2013 Recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin. Infect. Dis. 57, e22–e121. 10.1093/cid/cit278 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

