Time to Detection of Growth for Mycobacterium tuberculosis in a Low Incidence Area
- PMID: 34490143
- PMCID: PMC8418320
- DOI: 10.3389/fcimb.2021.704169
Time to Detection of Growth for Mycobacterium tuberculosis in a Low Incidence Area
Abstract
Background: Diagnosis of Mycobacterium tuberculosis (MTB) infection can be confirmed by Xpert assays within hours. However, when sample size does not allow performing both culture and Xpert, or if Xpert is negative, then formal diagnosis of MTB relies on culture and time to detection of growth (TDG) becomes critical for clinical management.
Objectives: To determine TDG in Xpert negative samples, or in samples in which Xpert could not be performed, in a low-incidence area for MTB.
Methods: Retrospective analysis (2015-2020) of a database including all cultures for mycobacteria in a University Hospital covering approximately 500'000 inhabitants. Analysis was restricted to culture positive (C+) samples for MTB for which 1/Xpert was negative or could not be performed because of limited sample volume, and 2/collected from subjects treated less than 24 hours. TDG was analyzed according to microscopy, origin of sample (pulmonary or not) and presence of cavitation.
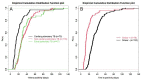
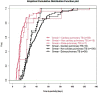
Results: Among 837 C+ samples for MTB, 236 samples (80% of respiratory origin) from 147 patients fulfilled study criteria; 78 samples (49 patients, 33%) were acid-fast bacilli (AFB) positive. Median (IQR) TDG was 25 (17; 40) days for all samples. TDG exceeded 28 days in 43% of samples and was significantly shorter in AFB+ vs AFB- samples, and samples from cavitary vs non cavitary or extra-thoracic disease.
Conclusions: In Xpert negative samples, or samples for which Xpert could not be performed, TDG exceeded 4 weeks in 43% of samples. AFB+ and samples from cavitary lung disease had a significantly shorter TDG.
Keywords: Mycobacterium tuberculosis; mycobacteria; mycobacterium growth indicator tube; nuclear acid amplification techniques; time to detection of growth.
Copyright © 2021 Vongthilath-Moeung, Poncet, Renzi, Schrenzel and Janssens.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bisognin F., Lombardi G., Lombardo D., Re M. C., Dal Monte P. (2020). Comparison of MycoPrep and the New MYCO-TB Kit: Rapid and Efficient Digestion and Decontamination of Respiratory Specimens for the Detection of Mycobacteria. New Microbiol. 43 (1), 13–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

